Environmental Engineering Reference
In-Depth Information
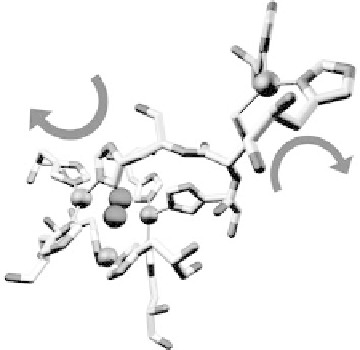
Figure 17.5 The protein environment around the Cu centers (gold spheres) of laccase from
Melanocarpus albomyces (PDB file 1GW0) showing a substrate O
2
molecule bound in the tri-
nuclear Cu site [Hakulinen et al., 2002]. The protein is depicted in stick representation with
atoms in their conventional coloring. (Courtesy of Armand W. J. W. Tepper.) (See color insert.)
transition that gives rise to an electronic absorption (l
max
600 nm) and the strong
blue color of the protein [Solomon et al., 1996]. Electrons move from this center
through a His-Cys-His electron transfer pathway to a trinuclear Cu cluster located at
a distance of about 1.3 nm. The substrate O
2
is bound at the trinuclear center in the
crystallographic structure represented in Fig. 17.5. In the electrochemical approach,
electrons for O
2
reduction are furnished by the electrode rather than by organic sub-
strates, and the “blue” Cu center must be in close contact with the electrode surface.
Laccase, a well-studied multi-copper oxidase, couples O
2
reduction to the serial
oxidation of four phenolic substrate molecules. In addition to applications in O
2
sen-
sing and fuel cell catalysis, the ability of laccase to oxidize a wide range of organic
molecules has led to widespread use of this enzyme in industrial catalysis, most nota-
bly in paper and textile bleaching and the bioremediation of wastewater [Couto and
Herrera, 2006]. The usefulness of laccases as cathode catalysts for enzyme fuel
cells depends on their catalytic efficiency k
cat
/K
M
and the midpoint potentials of
the active sites. Laccases are categorized into two classes on the basis of the potentials
of the “blue” Cu sites: for high potential laccases, the midpoint potential is about 680 -
790 mV, while for low-potential laccases, it is around 450 - 530 mV and limits the
range of organic substrates that can be oxidized. The nature of the amino acid residue
at the axial (fourth) position of the “blue” Cu center appears to strongly influence
the reduction potential. Strong ligands favor Cu
II
over Cu
I
, whereas a weakly ligating
residue (or no ligation at all) at this position destabilizes Cu
II
with respect to Cu
I
(con-
sistent with the tendency of Cu
I
to favor a three-coordination environment) and leads
to an increase in the reduction potential [Solomon et al., 1996]. This reasoning is not
sufficient, however, to explain the large difference in midpoint potentials between the
high and low potential laccases. Replacing the axial nonligating phenylalanine with a
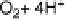


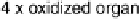










Search WWH ::

Custom Search