Environmental Engineering Reference
In-Depth Information
Figure 17.4 Cartoon representation of strategies for studying and exploiting enzymes on elec-
trodes that have been used in electrocatalysis for fuel cells. (a) Attachment or physisorption of an
enzyme on an electrode such that redox centers in the protein are in direct electronic contact with
the surface. (b) Specific attachment of an enzyme to an electrode modified with a substrate,
cofactor, or analog that contacts the protein close to a redox center. Examples include attachment
of the modifier via a conductive linker. (c) Entrapment of an enzyme within a polymer contain-
ing redox mediator molecules that transfer electrons to/from centers in the protein. (d)
Attachment of an enzyme onto carbon nanotubes prepared on an electrode, giving a large sur-
face area conducting network with direct electron transfer to each enzyme molecule.
Cu centers, for example. Provided that the relay center closest to the surface of the
protein makes good contact with the electrode (less than about 1.2 nm), so that inter-
facial electron transfer is fast, the electrocatalytic current measured will generally
reflect the rate of turnover at the active site. In many cases, direct adsorption of the
enzyme onto a pyrolytic graphite edge (PGE) electrode or a gold electrode modified
with an alkanethiolate monolayer provides a highly electroactive film (Fig. 17.4a).
Physisorption relies upon noncovalent interactions: hydrogen bonding, electrostatics,
hydrophobic bonding, and van der Waals forces. Studies of enzymes in this configur-
ation are termed protein film voltammetry [L´ger et al., 2003]. This strategy has been
particularly useful in studies and exploitation of H
2
oxidation and evolution by hydro-
genases, which comprise an electron relay chain of iron - sulfur clusters leading from
the surface to the deeply buried active site (see Section 17.3.2) [Vincent et al., 2007].
In this configuration, all enzyme molecules are exposed to the electrolyte solution,
ensuring optimal accessibility of fuel or oxidant to the active site. Disadvantages of
this approach are the low density of catalytic sites achieved, due to the large size of
redox enzymes, and lack of control over the orientation of an enzyme, meaning that
some molecules are incorrectly positioned for fast electron transfer. In some cases,
addition of a charged co-adsorbate helps to stabilize an adsorbed enzyme or to
favor correct orientation (for specific examples, see Sections 17.2 and 17.3).
Covalent chemical attachment of an enzyme to an electrode or a polymer coating
requires mild and specific chemistry to react with amino acids in the protein in aqueous

































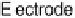


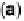
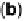
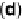


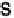








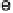

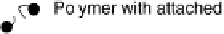














Search WWH ::

Custom Search