Environmental Engineering Reference
In-Depth Information
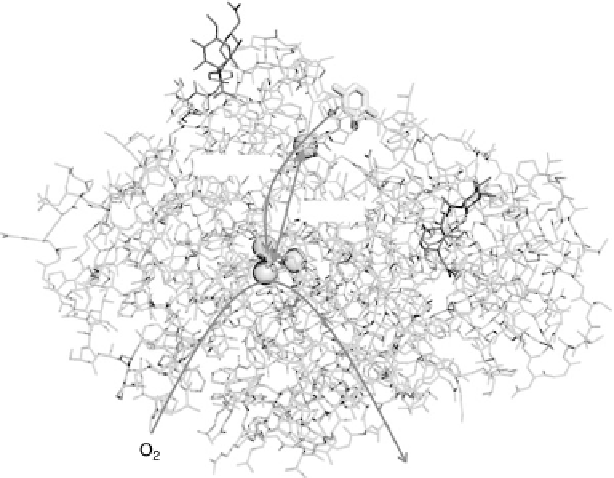
Figure 17.3 Anatomy of a redox enzyme: representation of the X-ray crystallographic struc-
ture of Trametes versicolor laccase III (PDB file 1KYA) [Bertrand et al., 2002]. The protein is
represented in green lines and the Cu atoms are shown as gold spheres. Sugar moieties attached
to the surface of the protein are shown in red. A molecule of 2,5-xylidine that
co-crystallized with the protein (shown in stick form in elemental colors) is thought to
occupy the broad-specificity hydrophobic binding pocket where organic substrates are oxidized
by the enzyme. Electrons from substrate oxidation are passed to the mononuclear “blue” Cu
center and then to the trinuclear Cu active site where O
2
is reduced to H
2
O. (See color insert.)
A paper by Heller and co-workers compares the electrocatalytic activity of “blue”
copper oxidases and Pt. Specifically, they have compared the overpotentials
required to reduce O
2
at 37 8C at a Pt fiber electrode operating in 0.5 M H
2
SO
4
and
an electrode involving laccase linked into a hydrogel containing Os redox centers
(see Section 17.2.1.1 for details of this assembly) and operating at pH 5 [Soukharev
et al., 2004]. Exact comparison is complicated by pH effects, but the laccase electrode
operates at a lower overpotential. Hydrogenases, like Pt, cycle H
2
with negligible over-
potential [Vincent et al., 2007]. As with laccase, it is difficult to gauge the absolute
activity, because the electroactive site density is rarely known. A rotating disk elec-
trode (RDE) modified with a nickel - iron hydrogenase has been shown to oxidize
H
2
at the same diffusion-controlled rate as a platinized electrode when both electrodes
are operated at 45 8C, 1 bar H
2
, pH 7 and 0.242 V versus SHE [Jones et al., 2002].
However, limiting interfacial electron transfer arising from poor coupling of some
enzyme molecules to the electrode meant that the platinized electrode was superior
at lower applied potentials. Other enzymes also exhibit electrocatalysis at negligible
overpotentials. For example, carbon monoxide dehydrogenase provides the only
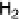


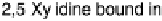











Search WWH ::

Custom Search