Environmental Engineering Reference
In-Depth Information
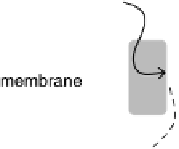
Figure 17.2 Bacterial cells harness energy from fuel oxidation coupled to oxidant reduction
in the form of a proton circuit. In aerobic bacteria such as Ralstonia species, H
2
oxidation in the
periplasmic compartment of the cell is coupled to O
2
reduction in the cytoplasm. Electrons are
transferred across the membrane by lipid-soluble electron-carrier quinol/quinone molecules,
and electron transfer is coupled to uptake of protons from the cytoplasm and their release
into the periplasm. This leads to a proton gradient across the membrane, analogous to charging
a capacitor in a fuel cell circuit (inset). A quinol oxidase makes a further contribution to the
proton gradient by pumping protons across the membrane as O
2
is reduced. The “load” on
this proton circuit is the transmembrane enzyme ATP synthase, which uses the proton gradient
to drive phosphorylation of ADP to generate ATP; hydrolysis of ATP elsewhere in the cell then
provides the energy to drive chemical reactions [Nicholls and Ferguson, 2002].
that operate under special conditions where traditional fuel cell catalysts fail. These
applications rely upon the high selectivity of enzymes for a substrate or group of
substrates, and the enormous variety of enzymes available in biology for specific
catalysis of a wide range of different reactions. Examples include devices that operate
on biologically derived fuels (e.g., sugars, glycerol, biomass, and sewage sludge),
contaminated fuels, or mixtures of fuel and oxidant. Fuel cells running on glucose
are particularly well developed. Research into enzyme electrocatalysis of O
2
reduction
and H
2
or methanol oxidation for fuel cells has been driven by a desire for low cost,
renewable alternatives to precious metal catalysts. The high turnover rates and
impressive selectivity of enzyme electrocatalysis have stimulated the development
of synthetic catalysts that mimic the structures of enzyme active sites (see, e.g.,
Liu et al. [2005]; Mahadevan et al. [2000]). Studies of enzyme electrocatalysis have
provided extensive information on reactions and mechanisms of redox enzymes.
Finally, application of enzymes in working fuel cells is providing information on




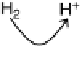


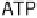



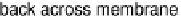







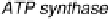
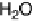

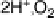



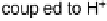



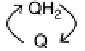









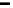



Search WWH ::

Custom Search