Environmental Engineering Reference
In-Depth Information
2.3 SIMPLE ION TRANSFER REACTIONS
In a simple electron transfer reaction, the reactant is situated in front of the electrode,
and the electron is transferred when there is a favorable solvent fluctuation. In con-
trast, during ion transfer, the reactant itself moves from the bulk of the solution to
the double layer, and then becomes adsorbed on, or incorporated into, the electrode.
Despite these differences, ion transfer can be described by essentially the same
formalism [Schmickler, 1995], but the interactions both with the solvent and with
the metal depend on the position of the ion. In addition, the electronic level on the reac-
tant depends on the local electric potential in the double layer, which also varies
with the distance. These complications make it difficult to perform quantitative
calculations.
As a reacting ion moves from the bulk of the solution towards the electrode sur-
faces, it loses a part of its solvation sheath, and therefore its energy increases.
Quantitatively, this effect is described by the potential of mean force (PMF), which
is the average potential energy that the ion experiences at a given position. We
focus here on the PMF due to the interactions with the solvent, which is the average
energy of interaction of the ions with the solvent at a given position. Good estimates
can be obtained by molecular dynamics simulations; for the simple case of an iodide
ion I
2
, the PMF is shown in Fig. 2.6 [Pecina et al., 1995]. In the bulk of the solution,
the PMF is constant; in the plot, it has been set to zero. When the ion starts to penetrate
the first layer of water in contact with the electrode, its energy rises, until it is in contact
with the electrode. The total rise is about 1.5 eV; the hydration energy of the ion in the
bulk is about 23 eV, so it loses roughly half of its solvation energy when it moves to
the surface.
Figure 2.6 Potential of mean force of an iodide ion in front of a Pt(111) electrode due to its
interaction with the solvent. The electrode is situated at x ¼ 0; the value in the bulk has been set
to zero. The data have been taken from Pecina et al. [1995].







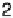
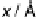
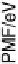



















Search WWH ::

Custom Search