Environmental Engineering Reference
In-Depth Information
formed, with the smallest particles (at lowest equivalent thickness) corresponding to
about 1 nm. These results are clearly consistent with the results obtained on high
area carbon-supported Pt, where a decrease in specific activity in 0.5 M H
2
SO
4
electrolyte was also observed [Guerin et al., 2004]. In both cases, the decrease in
oxygen reduction activity is accompanied by a concomitant shift in the Pt surface
reduction potential as particle sizes decrease [Guerin et al., 2004].
16.6 CO ELECTRO-OXIDATION ON SUPPORTED Au
AND Pt NANOPARTICLES
As for the reduction of oxygen, supported Au particles have been tested for their ability
to catalyze oxidation of carbon monoxide in HClO
4
. Figure 16.9 shows the electroche-
mical responses of eight electrodes out of arrays of electrodes of either carbon- or titania-
supported Au nanoparticles. The measurements were carried out in CO-saturated 0.5
M HClO
4
. Each of the four pairs of voltammograms corresponds to surfaces
supporting similar Au particle sizes on carbon and titania, allowing a direct compari-
son of the influence of the support. Results for particles between about 2.5 and 7.5 nm
are shown. While CO oxidation is seen at all the surfaces studied, and the peak current
Figure 16.9 Comparison of cyclic voltammetry in a CO-saturated electrolyte (0.5 M HClO
4
)
of Au supported on carbon (solid curves) and titania (dashed curves) for four different particle
sizes (indicated). The measurements were made at a temperature of 298K and a scan rate of
50 mV s
21
.






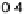
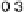



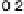



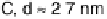
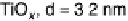
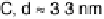
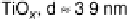
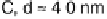
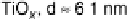
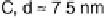

























































Search WWH ::

Custom Search