Environmental Engineering Reference
In-Depth Information
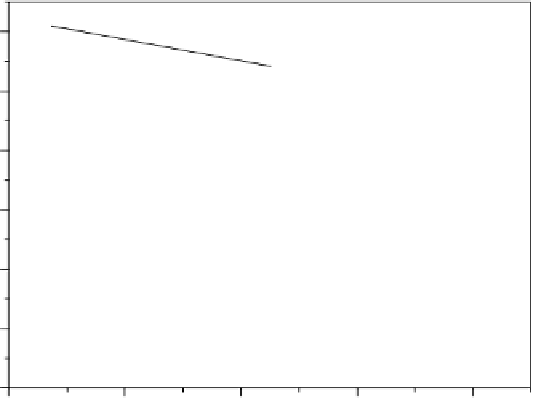
Figure 15.7 Logarithm of the kinetic current for the ORR in oxygen-saturated liquid electro-
lytes versus inverse diameter for Pt particles supported on Vulcan XC-72: (1) 0.9 V vs. RHE at
60 8S [Gasteiger et al., 2005]; (2) 0.85 V vs. RHE at room temperature [Maillard et al., 2002];
(3) 0.85 V vs. SHE at room temperature [Guerin et al., 2004]. For curves 1 and 2, measurements
were performed with the “thin-layer” RDE in 0.1 N HClO
4
; for curve 3, they were performed
with stationary voltammetry in 0.5 N H
2
SO
4
. (Curves have been replotted from Maillard et al.
[2002]; Gasteiger et al. [2005], Copyright 2002 and 2005, with permission from Elsevier; and
from Guerin et al. [2004], Copyright 2004 American Chemical Society.)
PO
32
and HSO
4
2
/SO
22
anions on Pt(111) planes, whereas adsorption is much weaker
on (110) and (100) planes. Recently, Kuzume and co-workers investigated the ORR
on [1
¯
1] stepped Pt single crystals [Kuzume et al., 2007]. In both H
2
SO
4
and
HClO
4
solutions, the Pt(111) surface showed the lowest catalytic activity among
those studied. In both H
2
SO
4
and HClO
4
, the plot of electrocatalytic activity versus
step density was nonlinear. In H
2
SO
4
, the activity increased strongly from Pt(111)
to Pt(221), but did not change for (331), (551), (771), and (110) planes. In HClO
4
,
the overall influence of crystalline structure on ORR electrocatalysis was much less
pronounced and the plot of activity versus step density exhibited a maximum. The
experimental results were rationalized in terms of the site blocking effect of sulfate/
bisulfate but the site blocking and energetic effect of adsorbed OH and surface
oxide. Kinoshita and co-workers have noticed that, qualitatively, the dependence of
MA on particle size followed the same trend as the mass averaged distribution
(where MAD(hkl) ¼ number of atoms in the (hkl) orientation/total number of
atoms) of (111) and (100) facets [Giordano et al., 1991; Kinoshita, 1990]. Indeed,
MAD(111) and MAD(100) reach maxima at particle sizes around 3.5 nm. If these





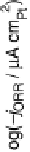






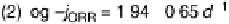



































Search WWH ::

Custom Search