Environmental Engineering Reference
In-Depth Information
Given that the HOR is one of the rare examples of “positive” size effects, it would
be desirable to verify this conclusion with planar model electrodes of simpler struc-
ture, where overlap of diffusion zones can be modeled in a straightforward manner.
If this is a true kinetic rather than a diffusion-related effect, it is important to under-
stand its origin and find out whether it is related to the decreased particle coverage
with H
UPD
as discussed in Section 15.4. It would also be interesting to know whether
it is related to changes in the metal nanostructure, namely transition from single-grain
particles at low metal loadings to multigrain structures at high loadings.
15.5.2 Oxygen Reduction Reaction (ORR)
The ORR is the most sluggish reaction in a PEMFC, and thus its understanding is very
important from both fundamental and application points of view:
O
2
þ
4e
þ
4H
þ
!
2H
2
O
E
o
¼
1
:
23 V vs. SHE at 298K (in acidic electrolytes)
(15
:
15)
A simplified scheme showing different pathways of the ORR, including the intermedi-
ate formation of H
2
O
2
, was proposed by Wroblowa, Bagotskii, and co-workers
[Wroblowa et al., 1976; Bagotskii et al., 1969] and is represented in Fig. 15.6. The
4e
2
path (k
1
) is usually called the direct or parallel pathway, while the one involving
intermediate H
2
O
2
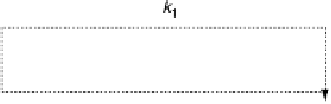
formation (k
2
, k
3
) is called the indirect or series pathway. For a
discussion of the ORR mechanism in greater detail, see Chapter 9. Despite numerous
investigations (see the reviews Tarasevich et al. [1983]; Damjanovic [1992]; Kinoshita
[1992]; Adzic [1998]; Gattrell and MacDougall [2003]; Durand et al. [1996] and
references therein), the mechanism of the ORR is still not fully understood.
Depending on the electrode material and the reaction conditions, various reaction inter-
mediates may be anticipated, in particular O
2,ad
,O
2,ad
,HO
2,ad
,O
ad
,OH
ad
, and H
2
O
2,ad
.
The last of these is the only stable intermediate; it can desorb from the electrode sur-
face and can be detected in the electrolyte. Determination of whether the series or the
Figure 15.6
Simplified scheme of the ORR [Wroblowa et al., 1976; Bagotskii et al., 1969].
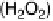







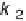
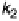
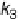
















Search WWH ::

Custom Search