Environmental Engineering Reference
In-Depth Information
in the weakly adiabatic range, a concept that we will discuss below: sufficiently strong
to ensure adiabaticity, but too weak to affect the energy of activation. Only very
recently has the role of the reactant - metal interaction, in particular the effect of
metal d-bands, been explored [Santos and Schmickler, 2007a, b, c].
In this chapter, we will review electrochemical electron transfer theory on metal
electrodes, starting from the theories of Marcus [1956] and Hush [1958] and ending
with the catalysis of bond-breaking reactions. On this route, we will explore the
relation to ion transfer reactions, and also cover the earlier models for noncatalytic
bond breaking. Obviously, this will be a tour de force, and many interesting side-
issues will be left unexplored. However, we hope that the unifying view that we
present, based on a framework of model Hamiltonians, will clarify the various aspects
of this most important class of electrochemical reactions.
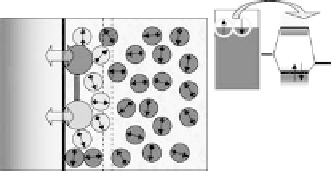
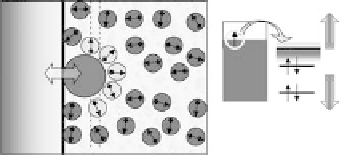
Figure 2.1 (Plate 2.1) shows a classification of the processes that we consider; they
all involve interaction of the reactants both with the solvent and with the metal elec-
trode. In simple outer sphere electron transfer, the reactant is separated from the elec-
trode by at least one layer of solvent; hence, the interaction with the metal is
comparatively weak. This is the realm of the classical theories of Marcus [1956],
Hush [1958], Levich [1970], and German and Dogonadze [1974]. Outer sphere trans-
fer can also involve the breaking of a bond (Fig. 2.1b), although the reactant is not in
direct contact with the metal. In inner sphere processes (Fig. 2.1c, d) the reactant is in
contact with the electrode; depending on the electronic structure of the system, the
electronic interaction can be weak or strong. Naturally, catalysis involves a strong
Figure 2.1 Classification of electrochemical electron transfer reaction on metal electrodes.
(See color insert.)



















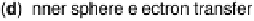






























Search WWH ::

Custom Search