Environmental Engineering Reference
In-Depth Information
Figure 15.4 Schematic representation of the Langmuir - Hinshelwood reaction between two
adsorbates: mobile X (black) and immobile Y (white) on a stepped single-crystalline surface
(a) and a facetted nanoparticle (b).
reactions. Let us consider a Langmuir - Hinshelwood (L - H) reaction between a
mobile (X) and an immobile (Y) adsorbate, occurring either on a stepped single crystal
(Fig. 15.4a) or on a nanoparticle (Fig. 15.4b). Let us assume that Y is adsorbed irre-
versibly either at a low coordinated site on the step, labeled (1), or at a high coordinated
site in the step (2). In the first case, X diffuses over the upper terrace and reacts with Y,
and, from the geometric standpoint, the active site configurations on a stepped single
crystal and at the nanoparticle edge will be similar. On the contrary, if Y is preferen-
tially adsorbed in the step, and the most favorable catalytic site is realized when X
approaches Y from the lower terrace, it is obvious that from the geometric standpoint
stepped single-crystal surface will offer a grossly different catalytic site geometry
compared with a facetted nanoparticle.
For example, Yates [1995] performed a detailed investigation of heterogeneous
catalytic CO oxidation at stepped Pt single crystals, and proposed that the reaction
occurs between an O atom in the step site with a neighboring CO molecule on the
lower terrace. This configuration was assumed to provide a favorable O
...
C22O
approach distance of about 0.25 nm. As discussed below, the difference in the site geo-
metries between steps and edges may to some extent be responsible for the different
behavior of stepped Pt single crystals and supported metal nanoparticles in electroche-
mical CO oxidation.
Last, but not least, one must not forget that steps and kinks appear as structural
defects on atomically flat surfaces of single crystals, while edges and vertices are
inherent structural elements of metal nanoparticles.
The general problem of model studies in aqueous electrolytes is related to anion-
specific adsorption. Kucernak and co-workers have compared the kinetics of the
HOR [Jiang and Kucernak, 2004] and the MOR [Jiang and Kucernak, 2005] on Pt
electrodes in H
2
SO
4
and in a solid state model cell of their own design, in which
Nafion
w
was used as the electrolyte. Differences were observed in the MOR kinetics,
and were related to suppression of OH
ads
formation in the aqueous electrolyte due to
the presence of sulfate/bisulfate anions. The anion effect can be reduced, but, unfor-
tunately, not fully eliminated, by using weakly adsorbing electrolytes, such as HClO
4
.
It should also be mentioned that the concentration and activity of water may differ sub-
stantially between model electrochemical cells with aqueous electrolytes and
PEMFCs. Meanwhile, recent spectroscopic studies have demonstrated the important




















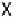








Search WWH ::

Custom Search