Environmental Engineering Reference
In-Depth Information
Figure 15.3 Simulated effectiveness factor for porous carbon electrode as a function of
the exchange current density j
0
and DC
O
for
j
h
j
¼ 0.4 V for a 10 wt% Pt/C catalyst layer
with g¼ 10, A ¼ 140 m
2
g
21
, r¼ 2gcm
23
, Nafion
w
volume fraction 0.6, thickness mm,
and ionic conductivity 0.05 S cm
21
. See the text for details. (Reproduced from Gloaguen
et al. [1994], with kind permission from Springer Science and Business Media.)
the flooded layer in the aqueous electrolyte is significantly lower compared with the
GDE owing to slow diffusion and low solubility of gases in the liquid phase
[Gloaguen et al., 1998]. As far as the exchange current density and the specific
electrocatalytic activity for the ORR are concerned, the values published in the
literature show significant scatter. For example, according to Wang and co-workers,
the intrinsic exchange current density for the ORR on Pt(111) is in the range 7 mA
cm
22
, j
0
, 25 mAcm
22
at room temperature in HClO
4
, with an intrinsic Tafel
slope from 2118 to 2130 mV dec
21
[Wang et al., 2004]. Gasteiger and co-workers
compared activities for polycrystalline Pt, Pt black, and Pt/C catalysts in HClO
4
at
0.9 V and 60 8C [Gasteiger et al., 2005]. Assuming a Tafel slope of 60 mV dec
21
in
the high potential region, the reported values for polycrystalline Pt translate into
j
0
20 nA cm
22
. Meanwhile, the values of the apparent rate constant for the ORR
at 0.8 V and 60 8C reported by Yano and co-workers [Yano et al., 2006] for
polycrystalline Pt and carbon-supported Pt nanoparticles after recalculation give
j
0
1nAcm
22
.



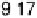



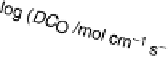




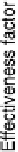

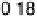

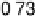



























Search WWH ::

Custom Search