Environmental Engineering Reference
In-Depth Information
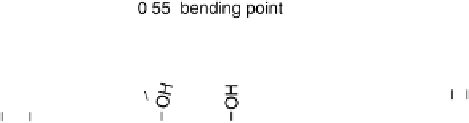
Figure 14.11 Illustration of CO electro-oxidation at Pt-modified Ru(0001): (a) mixed, non-
reactive adlayer; (b) Pt-assisted formation of OH
ad
at high local adsorbate coverages on the
Ru areas; (c) CO oxidation at the Pt islands. For simplicity, H
þ
is used instead of H
3
O
þ
.
no OH
ad
or O
ad
will be formed on the Pt islands themselves (see also the base
voltammograms).
The sudden increase in slope of the j - E curves at the bending points in the positive-
going scans is most simply explained by a change in the dominant reaction pathway,
which may either be directly induced by the potential or by potential-induced modi-
fications of the adlayer composition. In the present case, we assume that, for potentials
negative of the bending points, both Pt and Ru sites take part in the CO oxidation pro-
cess, while at more positive potentials, CO oxidation on the Pt monolayer islands
becomes dominant (Fig. 14.11c). This tentative assignment agrees with conclusions
based on the Tafel slopes b of the j - E curves, which we estimate to be b . 300
meV/decade and b
120 meV/decade for the regions cathodic and anodic of the
bending points, respectively. Values of b
120 meV/decade are expected for path-
ways involving adsorbed intermediates, whose coverage are very high and thus vary
only weakly with potential [Trasatti, 2003], as expected for the densely packed,
mixed CO
þ
OH
ad
/O
ad
adlayers on the Pt-free Ru(0001) areas. The lower value of
the Tafel slope at potentials positive of the bending points is much closer to those
reported for CO electro-oxidation on Pt electrodes [Santos et al., 1991; Lebedeva
et al., 2000; Shubina et al., 2004], in agreement with our assumption that in this poten-
tial region, CO oxidation takes place predominantly at Pt sites.
Similar to bulk Pt electrodes, we correlate the onset potential for CO oxidation on
the Pt islands, at potentials close to the bending points, with the formation of OH
ad
/
O
ad
species on these areas (see the base CV in Fig. 14.4), possibly at island edge sites,
in competition with CO adsorption. The subsequent reaction between CO
ad
and OH
ad
is apparently facile under these conditions. Since the binding energies of CO
ad
[Schlapka et al., 2003] and OH
ad
/O
ad
[Lischka et al., 2007] to pseudomorphic Pt
layers on Ru(0001) change with the thickness of the Pt film, the onset potential for
CO oxidation should also change with Pt film thickness. This explains why for the
0.9 ML Pt electrode the bending points in the positive-going and negative-going
scans, and the maximum in the positive-going scan, are shifted to lower potentials
compared with the surfaces with lower Pt contents: At 0.9 ML Pt, about 10% of the
surface is covered by second-layer islands on top of the monolayer Pt film.
Considering preliminary measurements on a 1.5 ML Pt-covered sample, which
show j - E curves comparable to those for 0.9 ML Pt, the presence of second-layer
islands seems to be decisive for the downshift of the bending point potential for CO






























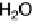




















































Search WWH ::

Custom Search