Environmental Engineering Reference
In-Depth Information
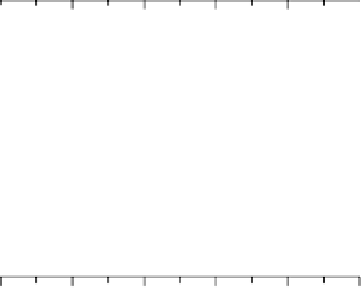
Figure 14.6 Charge in the cathodic peak between 0.11 and 0.06 V as a function of Pt surface
content: diamonds, Pt
x
submonolayers on Ru(0001); circles, Pt
x
Ru
12x
/Ru(0001) surface alloys;
the lines are predicted trends for linear or polynomial correlations between charge and Pt surface
content.
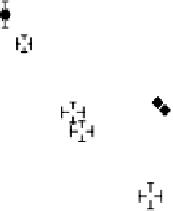
(H
ad
) or more strongly (O
ad
/OH
ad
) bound. In Fig. 14.7, we illustrate the resulting
potential-dependent adlayer formation and replacement processes for anodic (upper
part) and cathodic (lower part) scan directions. In the negative-going scan, H
upd
formed on the Pt islands can react with OH
ad
on neighboring Ru sites and desorb
as H
2
O [equivalent to Reaction (14.1a)]. Spillover of further H
upd
from the Pt islands
to the Ru terraces or direct adsorption of H
upd
on the Ru areas results in further OH
ad
removal and subsequent replacement by H
ad
. The pronounced shift of peak A
0
from
Figure 14.7 Illustration of the formation, removal, and exchange of adlayers on Ru(0001) in
the presence of Pt islands/sites as observed in the peaks A/A
0
,B/B
0
, and C/C
0
(see also
Figs. 14.2 and 14.8). Processes in the anodic/cathodic potential scan direction are shown in
the upper/lower part; for simplicity, H
þ
is used instead of H
3
O
þ
.
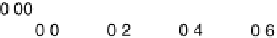























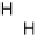






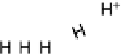

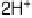



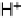
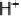

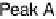

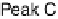


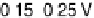


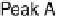
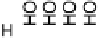
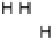



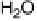



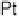
























































































Search WWH ::

Custom Search