Environmental Engineering Reference
In-Depth Information
Figure 13.9 Reaction scheme for C
1
molecule oxidation on a Pt/C catalyst electrode, includ-
ing reversible diffusion from the bulk electrolyte into the catalyst layer, (reversible) adsorption/
desorption of the reactants/products, and the actual surface reactions. The different original
reactants (educts) and products are circled. For removal/addition of H, we do not distinguish
between species adsorbed on the Pt surface and species transferred directly to neighboring
water molecule (H
ad
,H
þ
); therefore, no charges are included (H
þ
, e
2
). For a description of
the individual reaction steps, see the text.
2004; Hartnig and Spohr, 2005], although the proposals for the reaction pathways
differ. In several studies, this was proposed to result from O - H bond breaking and
subsequent transfer of a C-bonded H atom to the Pt substrate [Greeley and
Mavrikakis, 2004]. Hartnig et al. predicted a mechanism in which the CH
3
OH
molecule is polarized by formation of a hydrogen bond between the OH hydrogen
and a neighboring water molecule. Upon adsorption (via the C atom), one H atom is
transferred to the Pt substrate, and the OH hydrogen is fully transferred to the neighbor-
ing water molecule, resulting in an adsorbed HCHO
ad
molecule [Hartnig and Spohr,
2005; Hartnig et al., 2007b]. Desorption of this species into the catalyst layer will
result in formaldehyde as an incomplete oxidation product, which can either re-adsorb
or finally diffuse into the bulk electrolyte. Based on the above theoretical studies, there
would be no “direct” pathway to CO
2
in addition to that via formation, possibly








































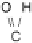

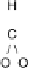




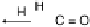











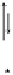


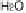



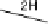













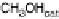

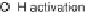
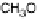














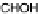






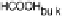
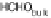
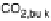

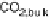
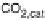



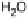


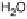




















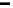























Search WWH ::

Custom Search