Environmental Engineering Reference
In-Depth Information
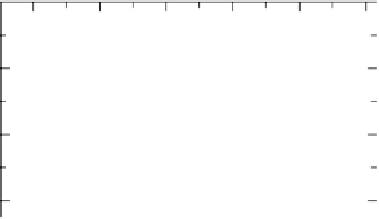
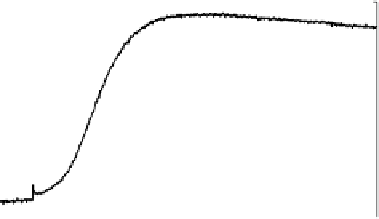
Figure 13.6 Potential-step electro-oxidation of formaldehyde on a Pt/Vulcan thin-film elec-
trode (7 mg
Pt
cm
22
, geometric area 0.28 cm
2
) in 0.5 M H
2
SO
4
solution containing 0.1 M HCHO
upon stepping the potential from 0.16 to 0.6 V (electrolyte flow rate 5 mLs
21
, at room tempera-
ture). (a) Solid line, faradaic current transients; dashed line, partial current for HCHO oxidation
to CO
2
; dotted line, difference between the net faradaic current and that for CO
2
formation. (b)
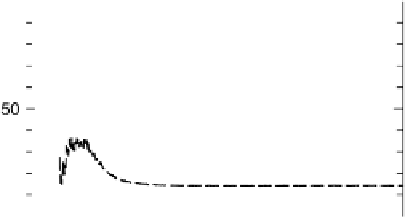
Solid line, m/z ¼ 44 ion current transients; gray line: potential-step oxidation of pre-adsorbed
CO derived upon HCHO adsorption at 0.16 V, in HCHO-free sulfuric acid solution. (c) Current
efficiency transients for CO
2
formation (dashed line) and formic acid formation (dotted line).
Figure 13.7 (Continued ) (b) Solid line, m/z ¼ 44 ion current transients; gray line, potential-
step oxidation of pre-adsorbed CO derived upon CH
3
OH adsorption at 0.16 V, in CH
3
OH-free
H
2
SO
4
solution. (c) m/z ¼ 60 ion current transients. (d) Current efficiency transients for CO
2
formation (dashed line), formic acid formation (dash - dotted line), and formaldehyde formation
(dotted line).
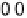
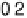
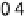





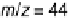


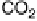
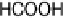















































































































Search WWH ::

Custom Search