Environmental Engineering Reference
In-Depth Information
formic acid. Hence, under the present reaction conditions, incomplete oxidation of
formaldehyde to formic acid prevails over complete oxidation to CO
2
.
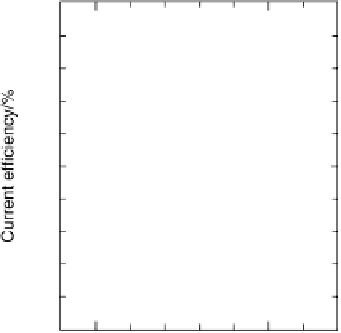
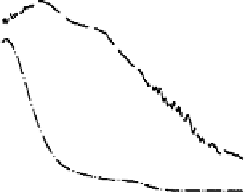
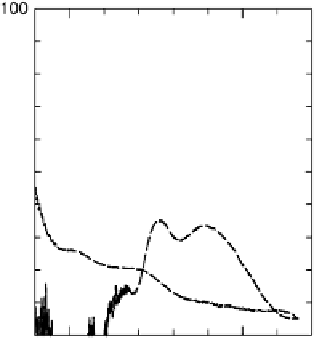
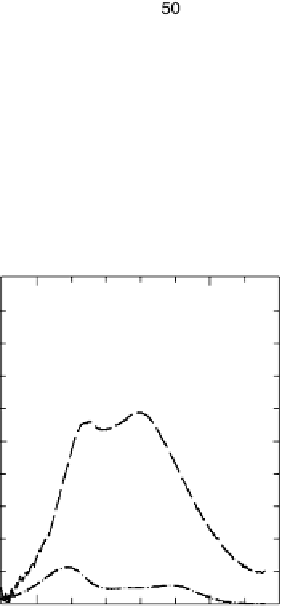
The current efficiencies for CO
2
formation and formic acid formation during poten-
tiodynamic formaldehyde oxidation, calculated from the data in Fig. 13.3b as the ratio
of the partial currents to the total faradaic current (in %), are plotted in Fig. 13.4a.
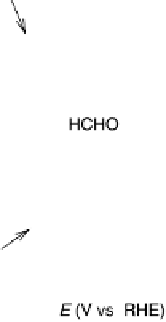
Figure 13.4 Current efficiency plots for the potentiodynamic electro-oxidation of formal-
dehyde (a) and methanol (b: positive-going scan; c: negative-going scan) on a Pt/Vulcan
thin-film electrode (data from Fig. 13.3a, b): dashed lines, current efficiency for CO
2
formation;
dash - dotted lines, current efficiency for HCOOH formation; dotted lines, current efficiency for
HCHO formation.

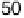
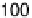


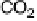


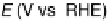


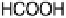
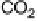
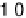
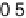

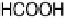
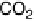
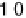
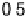

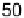
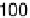















































































































































































































































































































































































































Search WWH ::

Custom Search