Environmental Engineering Reference
In-Depth Information
Figure 13.3 Potentiodynamic electrooxidation of (a) formic acid, (b) formaldehyde, and
(c) methanol on a Pt/Vulcan thin-film electrode (7 mg
Pt
cm
22
, geometric area 0.28 cm
2
)in
0.5 M H
2
SO
4
solution containing 0.1 M HCOOH (a), HCHO (b), or CH
3
OH (c). The potential
scan rate was 10 mV s
21
and the electrolyte flow rate was 5 mLs
21
, at room temperature). The
top panels show the faradaic current (solid lines), the partial currents for C
1
oxidation to CO
2
(dashed lines) and for formic acid formation (dash-dotted line), calculated from the respective
ion currents, and the difference between the measured faradaic current and the partial current for
CO
2
oxidation (formic acid oxidation (a), formaldehyde oxidation (b)), or the difference
between faradaic current and the sum of the partial currents for CO
2
formation and formic
acid oxidation (methanol oxidation, (c)) (dotted line). The solid lines in the lower panels in





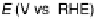























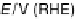





































































































































































































































































































































































































Search WWH ::

Custom Search