Environmental Engineering Reference
In-Depth Information
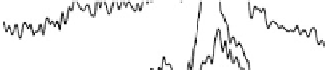
Figure 12.20 Potential-dependent SFG spectra from atop and multiply bonded CO on a
Pt(111)/Ru electrode in 0.1 M H
2
SO
4
at 1 mV/s (see Fig. 12.18). The scan potential for
each spectrum is shown on the right. Data show disappearance of atop CO at lower potentials
than multiply bonded CO.
sites at higher potential than the atop CO—namely, to nearly 0.30 V, in agreement
with the voltammetric stripping behavior of Fig. 12.18. This shows that the bridge-
bonded CO is more stable with respect to the electro-oxidation reaction than the
atop CO (and the 3-fold CO [Lagutchev et al., 2006]). In contrast, both bridge-
bonded and atop CO disappear simultaneously from pure Pt(111) (without Ru), as
shown in Fig. 12.11a and in support of the data reported before [Friedrich et al., 2002].
12.5.2 Vibrational, Electrochemical, and Electronic Properties
of the Pt
/
Ru Catalyst
The Pt/Ru catalyst is the material of choice for the direct methanol fuel cell (DMFC)
(and hydrogen reformate) fuel cell anodes, and its catalytic function needs to be com-
pletely understood. In the first approximation, as is now widely acknowledged, metha-
nol decomposes on Pt sites of the Pt/Ru surface, producing chemisorbed CO that is
transferred via surface motions to the active Pt/Ru sites to become oxidized to CO
2

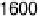

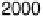
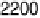
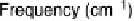



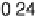
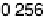
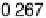











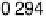
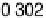
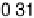




















Search WWH ::

Custom Search