Environmental Engineering Reference
In-Depth Information
probe of surface coverage. As described above, the transition causes both the
vibrational and the electronic polarizability to change, so none of SFG, SHG, or
IRAS alone can quantitatively determine coverage changes across this phase bound-
ary. Combining these measurements with (12.6) allowed accurate measurements of
surface coverage [Lagutchev et al., 2006]. A small (about 20%) discrepancy in the
SFG determination of atop coverage was attributed to either a small amount of surface
disorder or uncertainties in the SHG and IRAS measurements.
12.4.3 Decomposition of Methanol on a Pt Electrode
Tadjeddine and co-workers have used SFG [Guyot-Sionnest and Tadjeddine, 1990;
Eisenthal, 1992; Richmond, 2002; Vidal et al., 2002, 2004, 2005] to study the
adsorbed CO produced from a variety of solution species, including methanol
[Vidal et al., 2002, 2005]. With BB-SFG, we studied the electrochemical kinetics
of methanol chemisorption as surface CO, as shown in Fig. 12.13. We used a polycrystal-
line Pt electrode and 0.1 M H
2
SO
4
electrolyte with 0.1 M methanol. Figure 12.13a - d
characterize the potential-dependent SFG spectra obtained under the voltammetric
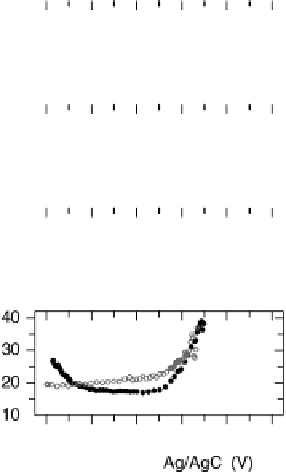
Figure 12.13 Electrochemistry and kinetics of CO resulting from methanol decomposition on
polycrystalline Pt with 0.1 M H
2
SO
4
electrolyte and 0.1 M methanol. (a - d) Current, SFG
amplitude, frequency, and width of adsorbed CO, scanning the potential in both directions as
indicated with the solid line and filled circles denoting the forward (anodic) scan and the
dashed line and unfilled circles denoting the back (cathodic) scan. (e - g) Starting at 0.6 V,
where the adsorbed CO is rapidly electro-oxidized, the potential is suddenly jumped to 0.2 V.
The reformation of the CO layer (CO chemisorption) due to methanol decomposition occurs
in about 20 s. The adsorbed CO molecules are redshifted, and have a broader spectrum at shorter
times, when the adlayer coverage is low.




















































Search WWH ::

Custom Search