Environmental Engineering Reference
In-Depth Information
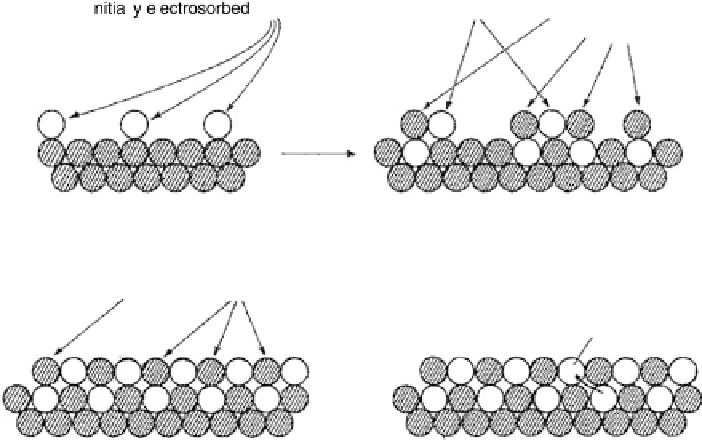
long-term potentiostatic build-up of surface oxide charge on Pt (in an inert atmos-
phere) was proportional to the logarithm of the time, and suggested the schematic
description shown in Fig. 1.4 for processes at the atomic scale on and in the Pt surface,
when holding the Pt sample at a constant positive potential for extended periods of
time. The figure is just a schematic; however, it can serve as a basis for consideration
of two important factors. One is identification of the origin of the irreversible reduction
of chemisorbed oxygen on Pt (and the Q
oxide
versus log (time) rate law documented) as
a “place exchange” process between chemisorbed oxygen atoms (or OH
ads
) and sur-
face Pt atoms. This type of process can be understood as an early step in the formation
of a three-dimensional oxide phase from the very initial state of oxygen chemisorption
on the metal surface. According to Conway et al. [1990], the place exchange process
described schematically in Fig. 1.4 results in the conversion of an initial sub-mono-
atomic layer of chemisorbed oxygen on top of a Pt metal surface into a structure of
mixed layers of Pt and oxygen atoms (or OH groups). In line with this reasoning,
the outer surface of a “Pt” catalyst in a PEFC cathode, particularly following long-
term exposure to high potentials at elevated temperatures, could in fact be a mixed
Pt/oxygen atomic layer.
Figure 1.4 also highlights the possibility of a significant range of Pt22OH bond
strengths, considering on one end of the spectrum the OH groups well surrounded
by metal atoms and, at the other end, OH groups on top of Pt surface atoms, likely cor-
responding to those “last formed” surface OH groups that have not undergone further
reorganization into a more stable sub-monolayer lattice of adsorbed intermediates, not
to mention insertion under the metal skin. The latter type of OH species was identified
Figure 1.4 Proposed steps in the chemisorption of OH on/in Pt, starting with arrays of OH
groups over the uppermost metal atom layer, increasing the coordination number of the adsorbed
OH by place exchange, and next generating a mixed, metal/oxygen overlayer while further
oxidizing to form O atoms. From Conway et al. [1990].
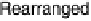

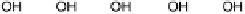

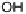

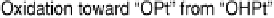
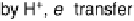
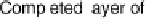
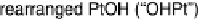

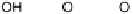


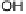
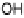
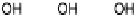
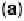
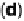
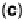
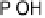









Search WWH ::

Custom Search