Environmental Engineering Reference
In-Depth Information
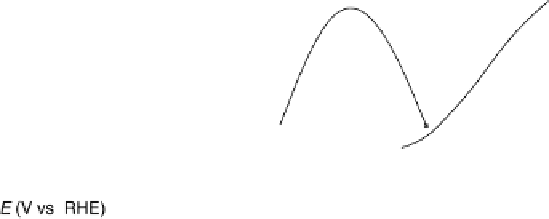
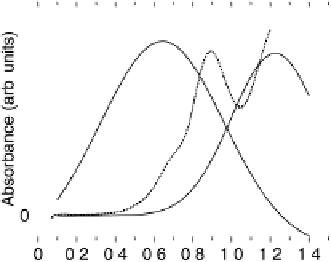
Figure 11.9 Intensities of the CO
L
(
A
) and CO
2
(
B
) bands as functions of potential. (a, b)
From the spectra of the species coming from methanol adsorption and oxidation (0.1 M
HClO
4
þ
0.1 M CH
3
OH, 25 8C): (a) Pt/C electrode; (b) Pt
0.8
þ
Ru
0.2
/C electrode. (c, d)
From the spectra of the species coming from ethanol adsorption and oxidation (0.1 M
HClO
4
þ
0.1 M C
2
H
5
OH, 25 8C): (c) Pt/C electrode; (d) Pt
0.9
Sn
0.1
/C electrode. The dashed
curves in (c) and (d) show I(E).
et al., 2004b]. Actually, this can be explained by the fact that modification of Pt by Sn
induces two important effects: first, it leads to a decrease in the yield of CO
2
(which is
twice as high with a Pt/C catalyst as with a Pt-Sn/C catalyst); second, it greatly favors
the formation of acetic acid compared with acetaldehyde, as was shown by analyzing
the reaction products in the anode outlet of a DAFC (Table 11.2) [Rousseau et al.,
2006]. The dilution of surface Pt sites by addition of Sn atoms is responsible for the
first effect. The introduction of Sn atoms between Pt atoms decreases, by a statistical
effect, the ability of the catalytic surface to cleave the C - C bond in the adsorption
reaction of ethanol (which needs at least three adjacent Pt atoms). The bifunctionnal
mechanism [Watanabe et Motoo, 1975a] is responsible for the second effect. In this
mechanism, ethanol is adsorbed dissociatively at Pt sites, either via an O-adsorption
or a C-adsorption process [Iwasita and Pastor, 1994a, b; Rightmire et al., 1964] to
form acetaldehyde species, according to the following reactions:
Pt
þ
CH
3
-CH
2
OH
!
Pt
(OCH
2
-CH
3
)
ads
þ
e
þ
H
þ
(11
:
13)








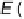





























































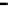


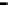

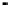

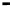








Search WWH ::

Custom Search