Environmental Engineering Reference
In-Depth Information
TABLE 11.1 TEM and XRD Characterization of Different Catalysts
Prepared by the Colloidal Route
a
Catalyst
Average Size/nm
Structure
Pt/C
2.2
fcc
Pt
0.8
þ
Ru
0.2
/C
2.1
Pt fcc
þ
Ru hc
Pt
0.8
Ru
0.2
/C
1.9
fcc (alloy)
Pt/C
þ
Ru/C (80 at% Pt
and 20 at% Ru)
Pt 2.1; Ru 1. 5
Pt fcc
þ
Ru hc
Ru/C
1.5
hc
a
Experimental details are given in Dubau et al. [2003a, b] and Dubau [2002].
is in agreement with the works of Waszczuk and Brankovic, who demonstrated an
electrocatalytic enhancement of methanol oxidation at Pt particles decorated by Ru
compared with the alloy compounds of the same composition [Waszczuk et al.,
2001b; Brankovic et al., 2002a, b].
The problem of the optimum Pt/Ru atomic ratio is of great interest. The differences
in activity as a function of Ru content are due to the balance between the initial step of
adsorption - dehydrogenation of methanol at Pt sites and the following step of
adsorbed CO species oxidation [Gasteiger et al., 1993]. Some authors claim that a
Pt/Ru atomic ratio of 50/50 is the best for methanol electro-oxidation [Dinh et al.,
2000; Watanabe et al., 1987]. Others propose that a ratio close to 80/20 is optimal
[Kabbabi et al., 1998; Gasteiger et al., 1993; Ianniello et al., 1994; Dubau et al.,
2003a]. The discrepancy in the literature likely comes from a lack of knowledge of
the surface composition. The work of Wieckowski's group on Pt-Ru catalysts prepared
by spontaneous deposition of Ru at Pt nanoparticles is very interesting in this context
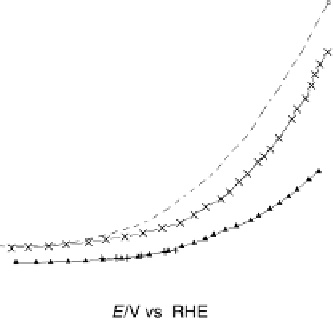
Figure 11.3 j(E) polarization curves for methanol oxidation on different colloid catalysts
prepared in different ways: (1) Pt/C; (2) Pt
0.8
Ru
0.2
/C; (3) Pt
0.8
þ
Ru
0.2
/C (0.5 M H
2
SO
4
,
1.0 M MeOH; sweep rate 1 mV s
21
, T ¼ 298K).
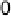
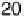
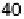

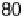






































Search WWH ::

Custom Search