Environmental Engineering Reference
In-Depth Information
Figure 11.1 Kinetics of adsorption of CO at a Pt catalyst from a 0.01 M methanol solution
at different potentials. (a) Pt black catalyst, with Pt loading 0.8 mg cm
22
. (b) Pt
0.5
Ru
0.5
black
catalyst, with catalyst loading 0.8 mg cm
22
(0.1 M H
2
SO
4
, T ¼ 298K).
catalyst structure [Dubau et al., 2003b; Waszczuk et al., 2001b; Brankovic et al.,
2002a, b]; optimization of the Pt/Ru atomic ratio [Kabbabi et al., 1998; Iwasita
et al., 2000; Dinh et al., 2000; Watanabe et al., 1987; Gasteiger et al., 1994;
Ianniello et al., 1994]; and the use of a third alloying metal [Lima et al., 2001]. A cru-
cial aspect is the method used to prepare nanostructured catalysts, leading to different
catalyst structures and compositions, in a controlled way by varying the experimental
conditions only slightly. The colloidal route for catalyst preparation derived from the
method developed by B ¨ nnemann and co-workers [B ¨ nnemann et al., 1991, 1996] is
very convenient for obtaining Pt-Ru compounds with controlled size, composition,
and structure [Dubau et al., 2003a, b; Dubau, 2002]. Catalytic powders can be
obtained in the following ways: (i) Pt/C and Ru/C, from deposition of a Pt colloid
or a Ru colloid solution on carbon; (ii) Pt-Ru/C, from deposition of alloyed Pt-Ru




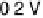

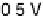
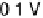

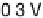






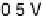
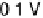

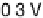






























































































































































































Search WWH ::

Custom Search