Environmental Engineering Reference
In-Depth Information
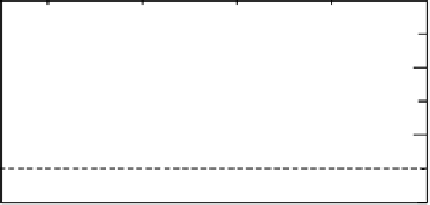
Figure 10.10 Temperature dependence of H
2
O
2
yield P(H
2
O
2
) at Nafion - Pt(4.8 nm)/CB
(W), Nafion2Pt(2.6 nm)/CB (D), Nafion2Pt(1.6 nm)/CB (S), Nafion - Pt(2.6 nm)/CB with
the same Pt loading as 1.6 nm catalyst (A), Nafion - Pt(bulk) (
†
), and Pt(bulk) without
Nafion coating (
5
). U
m
¼ 50 cm s
21
. (From Yano et al. [2006b], reproduced by permission
of the PCCP Owner Societies.)
Nafion) [Wakabayashi et al., 2005a], the Nafion coating on Pt is the major reason for
triggering H
2
O
2
production in a practical potential region E . 0.70 V. It has been
reported that DQ
H
at Nafion - Pt(bulk) decreases by about 10% compared with a
Pt(bulk) (without Nafion) electrode [Lawson et al., 1988; Chu et al., 1989; Zecevic
et al., 1997; Chu, 1998]. This reduction is probably due to a specific adsorption of sul-
fonate groups in Nafion, as observed by in situ FTIR spectroscopy [Ayato et al., 2006].
Therefore, sulfonate groups in Nafion could be the species strongly adsorbed on Pt sur-
face, modifying the surface property.
10.3.1.2 Arrhenius Plots of k
app
The value of I
k
at a given potential E is deter-
mined in the same manner as described in Section 10.2.4. Because the contribution of
two-electron reduction to the production of H
2
O
2
(compared with the overall ORR)
was very low, we can evaluate an apparent rate constant k
app
at a constant applied
potential from the following equation [Wakabayashi et al., 2005a, b]:
I
k
4FS
Pt
¼
k
app
[H
þ
][O
2
]
(10
:
8)




















































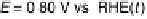














Search WWH ::

Custom Search