Environmental Engineering Reference
In-Depth Information
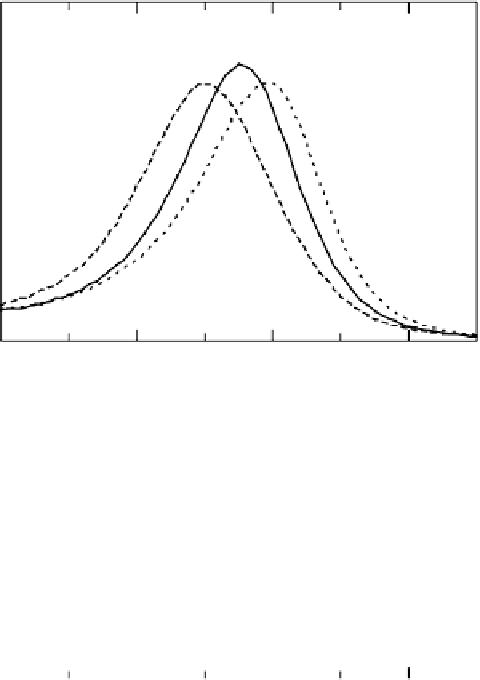
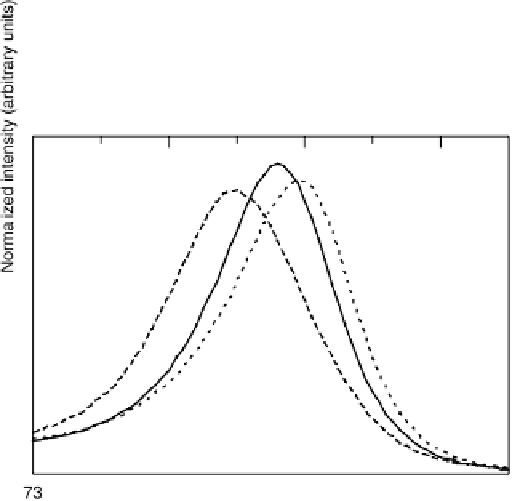
Figure 10.4 Area-normalized CL spectra of Pt4f
7/2
for the pure Pt (dotted line), Pt
58
Co
42
(solid line), and Pt
60
Ru
40
(dashed line) alloys with respect to E
F
: (a) as-prepared; (b) after elec-
trochemical stabilization. The samples were thin film pure Pt or Pt-based alloys (diameter 8 mm
and thickness 80 nm) prepared on Au disks by DC sputtering. Electrochemical stabilization of
Pt
58
Co
42
was performed by repeated potential cycling between 0.075 and 1.00 V at a sweep rate
of 0.10 V s
21
in 0.1 M HClO
4
under ultrapure N
2
(99.9999%) until CV showed a steady state.
Pt
60
Ru
40
was stabilized by several potential cycling between 0.075 and 0.80 V at 0.10 V s
21
in
0.05 M H
2
SO
4
under ultrapure N
2
. (From Wakisaka et al. [2006], reproduced by permission of
the American Chemical Society.)
It was found that the intensity of Co2p
3/2
decreased significantly (by a factor of
2.5), supporting the concept of Co dissolution from the alloy and formation of the
Pt skin layer on the electrode surface during electrochemical stabilization. As
shown in Fig. 10.4b, a clear CL shift was still observed in the Pt4f
7/2
spectrum for
the stabilized Pt-Co, in spite of the dissolution of Co, although the CL shift after stabil-
ization was slightly smaller (0.15 eV) than in the as-prepared alloy (0.19 eV). Thus, we


















Search WWH ::

Custom Search