Environmental Engineering Reference
In-Depth Information
was prepared from reagent grade chemicals and Milli-Q water and purified in
advance with conventional pre-electrolysis methods [Watanabe and Motoo, 1975b;
Uchida et al., 1997].
Before the measurement of HOR activity, a pretreatment of the alloy electrode was
carried out by potential sweeps (10 V s
21
) of 10 cycles between 0.05 and 1.20 V in
N
2
-purged 0.1 M HClO
4
. The cyclic voltammograms (CVs) at all the alloys resembled
that of pure Pt. As described below, these alloy electrodes were electrochemically
stabilized by the pretreatment. Hydrodynamic voltammograms for the HOR were
then recorded in the potential range from 0 to 0.20 V with a sweep rate of 10 mV s
21
in 0.1 M HClO
4
saturated with pure H
2
or 100 ppm CO/H
2
at room temperature.
The kinetically controlled current I
k
for the HOR at 0.02 V was determined from
Levich - Koutecky plots [Bard and Faulkner, 1994].
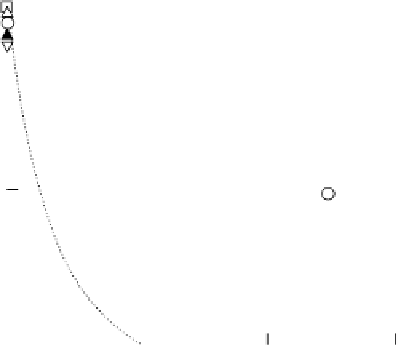
Figure 10.1 shows I
k
at various electrodes as a function of CO poisoning time at
26 8C. For the pure Pt electrode, the value of I
k
decreases and reaches nearly zero
after 30 minutes. In contrast, the Pt-Fe, Pt-Ni, Pt-Co, and Pt-Mo alloys retain
high HOR activity for a prolonged period of time; the reduction in I
k
is negligibly
small. Such CO tolerance of these alloys was found to be almost independent of the
composition; for example, alloying Pt with only 5 at% Fe resulted in excellent tol-
erance. However, Pt alloys with Ti, Cr, Cu, Ge, Nb, Pd, In, Sb, W, Au, Pb, or Bi
showed complete CO poisoning after a short time, while the combination of Pt
with Mn, Zn, Ag, or Sn exhibited only limited CO tolerance.
Figure 10.1 Time courses of kinetically controlled currents I
k
for the HOR at 0.02 V and
26 8C on various electrodes in 0.1 M HClO
4
saturated with 100 ppm CO (H
2
balance). CO was
adsorbed on each electrode at 0.02 V under the rotation rate of 1500 rev min
21
. (From Igarashi
et al. [2001], reproduced by permission of the PCCP Owner Societies.)

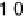

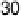
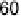


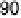

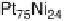
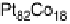
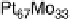
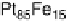































Search WWH ::

Custom Search