Environmental Engineering Reference
In-Depth Information
this mechanism [Bett et al., 1976]. They instead proposed a “2D Ostwald ripening”
mechanism involving surface transport of Pt atoms (not soluble Pt species) from
one crystallite to another. In such a mechanism, the particle size distribution would
broaden and shift toward larger particles owing to the consumption of smaller par-
ticles. However, a bimodal particle size distribution was observed during potential
cycling in some cases where some small Pt particles remained [Garzon et al., 2006;
More et al., 2006; Xie et al., 2005a, b], suggesting a combination of crystallite coalesc-
ence and Ostwald ripening processes [Borup et al., 2007].
9.4.3 Stabilization Effects of Au Clusters on Pt Electrocatalysts
Transportation is expected to be an especially important application of fuel cells, since
their uniquely high energy conversion efficiency may result in a substantial decrease in
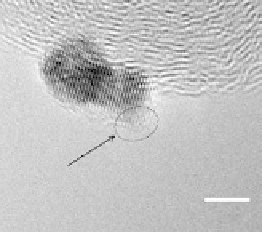
Figure 9.25 (a, b) Voltammetry curves for (a) Pt/C and (b) Au/Pt/C catalysts before and
after 30,000 cycles; the sweep rate was 50 and 20 mV/s, respectively. The potential cycles
were from 0.6 to 1.1 V in an O
2
-saturated 0.1 M HClO
4
solution at room temperature. For all
electrodes, the Pt loading was 1.95 mg (10 nmol) Pt on a 0.164 cm
2
glassy carbon rotating
disk electrode. The shaded area in (a) indicates the lost Pt area. (c) Au-modified Pt. Different
interference fringes (circled) indicate clusters of Au on Pt. (d) Catalytic activities of Pt/C and
Au/Pt/C before and after 30,000 cycles (normalized to geometric area). (Reproduced with per-
mission from Bi et al. [2007] and Zhang et al. [2007b].)





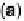
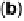
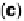
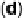
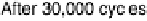




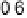

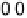

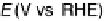
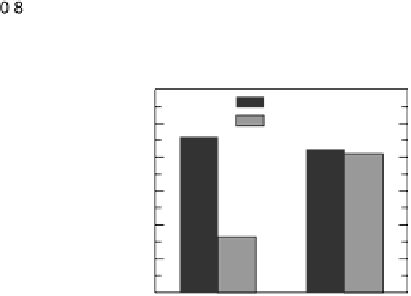

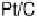


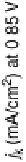





























Search WWH ::

Custom Search