Environmental Engineering Reference
In-Depth Information
reactivity of the surface [Shao et al., 2007a, b; Suo et al., 2007]. Compressive strain
alone accounts for a 0.1 eV destabilization of the Pd - O bond. Pd
3
Fe(111) and
PdFe(111) substrates contribute further destabilization of O by about 0.25 and
0.35 eV, respectively. The native Pd
3
Fe(111) and PdFe(111) surfaces bind oxygen
about 1 eV more strongly than equilibrium Pd, and are therefore not expected to be
good ORR catalysts. Of these surfaces, Pd skin on Pd
3
Fe(111) is most similar to
Pt(111) in terms of both the d-band center position and the oxygen binding energy,
which corroborates the findings in Fig. 9.21.
Similarly to the Pt monolayer catalysts, a series of Pd monolayers deposited on differ-
ent metal single crystals were tested for ORR activity. The results are shown in Fig. 9.22.
The ORR activity increases in the order Pd
ML
/Ru(0001) , Pd
ML
/Ir(111) , Pd
ML
/
Rh(111) , Pd
ML
/Au(111) , Pd(111) , Pd
ML
/Pt(111) , Pt(111). The activity of
the Pd
ML
/Pt(111) surface is higher than that of Pd(111), but somewhat lower than
that of Pt(111).
Some thermodynamic guidelines have been proposed for designing non-Pt alloy
ORR electrocatalysts. Bard and co-workers suggested that for Pd-M alloys, the reac-
tive metal M constitutes the site for breaking the O - O bonds, forming O
ads
that
migrates to the hollow sites dominated by Pd atoms, where it is readily reduced to
water [Fernandez et al., 2005a, b, 2006]. Based on this mechanism, the alloy surface
should consist of a relatively reactive metal such as Co, and the atomic ratio of this
metal should be 10 - 20% so that there are sufficient sites for reactions of O - O
bond breaking on M and O
ads
reduction at hollow sites formed by Pd atoms. DFT
calculations indicated that one of the O atoms diffused to the Pd hollow site while
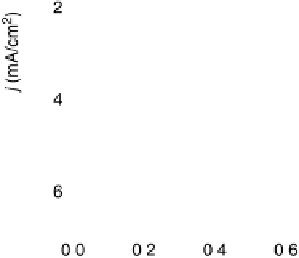
Figure 9.22 Comparison of polarization curves for the ORR on Pd monolayers on different
substrates, and on Pd(111) and Pt(111), in 0.1 M HClO
4
solution; sweep rate 10 mV/s; room
temperature. (Reproduced with permission from Shao et al. [2006a].)



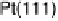
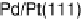

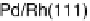
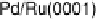

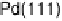






















Search WWH ::

Custom Search