Environmental Engineering Reference
In-Depth Information
The resulting free energy diagram at zero overpotential and the activity-and-barrier
plots (Fig. 9.20) (Plate 9.1) indicate that reductive adsorption (DG
RA
¼
0
:
46 eV) is
apparently not the RDS for the ORR on Pt, because dissociative adsorption
(DG
DA
¼
0
:
26 eV) offers a more favorable pathway at high potentials. This is at
variance with the conclusions in [Sidik and Anderson, 2002]. Above the reversible
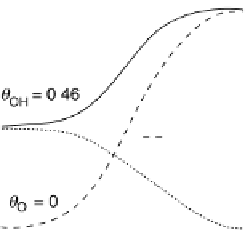
potential for O
!
OH, the O coverage is high, causing severe inhibition of
ORR activity. As the potential decreases, the O coverage falls, making the sites
available for OH adsorption and the kinetic current increases with the decreasing
reductive desorption activation barrier. Nonetheless, the activation barriers for both
O
!
OH (DG
RT
¼
0
:
50 eV) and OH desorption (DG
RD
¼ 0.45 eV) are high, causing
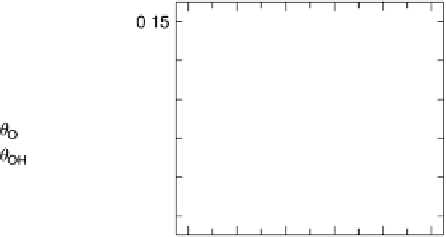
Figure 9.20 (a) ORR polarization curve for Pt(111) in 0.1 M HClO
4
solution. The fitted par-
ameters were used to calculate the adsorption isotherms for O and OH in (b) and to construct the
free energy diagrams in (c), where the lengths of the vertical lines represent the activation free
energies of the forward (solid) and backward (dashed) reactions. (d) Activity-and-barriers plot
that shows the kinetic current (black line) and the activation free energies (colored lines) on an
equivalent energy scale. Above the reversible potential for O
!
OH transition (0.87 V), the
apparent Tafel slope is half of that at low potentials. (Reproduced with permission from
Wang JX et al. [2007].)




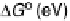



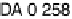
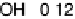



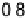
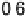
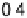


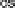

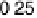
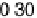
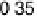
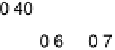
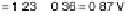




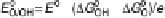

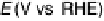










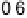


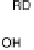
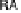

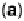
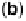

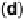








































































Search WWH ::

Custom Search