Environmental Engineering Reference
In-Depth Information
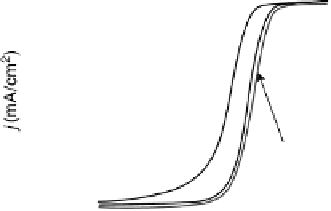
Figure 9.18 (a) Polarization curves for the ORR on Pd/C (10 nmol), Pt/C (10 nmol),
and Pt
ML
/Pd/C nanoparticles (10 nmol Pd) in 0.1 M HClO
4
solution. The electrode
geometric area was 0.164 cm
2
. The rotation rate was 1600 rev/min and the sweep rate was
10 mV/s. (b) The Pt and total noble metal mass activities for the ORR of Pt/C (10 nmol),
Pt
ML
/Pd/C (10 nmol Pd), and (Ir
0.2
Pt
0.8
)
ML
/Pd/C and (Re
0.2
Pt
0.8
)
ML
/Pd/C (20 nmol Pd)
nanoparticles expressed as a current at 0.8 V. (Reproduced with permission from Vukmirovic
et al. [2007].)
Pt
ML
/Pd/C and Pt/C are 1.7 and 12 mg
Pt
/cm
2
, respectively. The amount of Pt corre-
sponding to Pt
ML
/Pd/C can be obtained by calculating the charge associated with the
deposition of a Cu monolayer at underpotentials (after correcting for the double-layer
charging) on Pd/C, assuming that there is a one-to-one ratio between the Cu and Pd
atoms. Therefore, even though Pt/C has a higher surface area, and a Pt loading that is
seven times larger, its ORR activity is lower than that of Pt
ML
/Pd/C.
When the ORR activity is compared on a Pt mass basis, Pt
ML
/Pd/C is eight times
more active than commercial Pt electrodes, whereas (Pt
0.8
Ir
0.2
)
ML
/Pd/C and
(Pt
0.8
Re
0.2
)
ML
/Pd/C are nearly 20 times more active than Pt/C (Fig. 9.18b). This
finding underscores the promise of monolayer-based electrocatalysts in significantly
reducing the amount of Pt in fuel cell electrodes. The positive comparisons with
Pt/C nanoparticles remain valid even when the total precious metal content (m
Pt
þ
m
M
þ
m
Pd
) is considered instead of Pt mass alone: the total noble metal mass activity
of Pt-Ir and Pt-Re monolayer electrocatalysts is 4- and 4.5-fold higher than Pt/C. This
is still very satisfactory, particularly since the main constituent of the ternary alloys is
Pd, which is considerably less expensive than Pt.
XANES and in situ voltammetry [Zhang et al., 2005b] experiments show that, in
the case of (Pt
0.8
Ir
0.2
)
ML
/Pd/C, Pt-OH formation is suppressed until potentials as
high as 1.17 V, which also supports the original hypothesis that an appropriate
choice of metal M can keep the Pt sites from being poisoned by OH by destabilizing
interactions, and thereby facilitating the ORR. For comparison, the onset of Pt-OH for-
mation on the Pt/C catalysts was found to be less than 0.6 V. Thus, it is likely that the
ternary nanoparticles are more stable under potential cycling regimes than Pt nanopar-
ticles, resulting in more sinter-resistant catalysts.



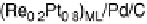








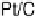



































Search WWH ::

Custom Search