Environmental Engineering Reference
In-Depth Information
Figure 9.16 ORR activity of two mixed-metal monolayer electrocatalysts supported on
Pd(111), expressed as the kinetic current density at 0.85 V as a function of the M : Pt ratio in
the Pd-supported Pt-M monolayer. (Reproduced with permission from Zhang et al. [2005b].)
In fact, for these two metals (M), adjacent OH groups react spontaneously to
yieldanH
2
O molecule and an O atom adsorbed on M.
When the kinetic current densities measured on the (Pt-M)
ML
/Pd(111) surface are
plotted against the effective repulsion energies (relative to the repulsion energy on
Pt
ML
/Pd(111)), an excellent linear correlation is found between the two variables
(Fig. 9.17b), which shows that the increased ORR activity of these Pt-M/Pd(111)
surfaces can be attributed to an increase in repulsive interaction between surface-
bound OH species, or, alternatively, an increased destabilization of OH species on
the Pt sites of the surface. This supports the original hypothesis that easily oxidized
metal atoms attract OH to them at lower potentials, thereby destabilizing OH on adja-
cent Pt sites and reducing OH lifetime and coverage on those sites. Therefore, DFT
calculations have been critical for the identification of this key reactivity descriptor
(OH - OH and OH - O interaction), which is responsible for reduced OH coverage
on these ternary alloy surfaces.
Additional experiments were carried out to study the behavior of Pd nanoparticles
coated with Pt or with Pt plus M, which more closely reflects the morphology of actual
catalyst particles. Figure 9.18a displays the polarization curves for the ORR on
commercial carbon-supported Pt nanoparticles (Pt/C), Pd nanoparticles (Pd/C), a
monolayer
of
Pt
on
Pd/C
(Pt
ML
/Pd/C),
and
mixed
(Pt
0.8
Ir
0.2
)
ML
/Pd/C
and



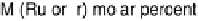














































Search WWH ::

Custom Search