Environmental Engineering Reference
In-Depth Information
Figure 9.14 Kinetic current density (squares) at 0.8 V for O
2
reduction on the Pt monolayer
deposited on various metal single-crystal surfaces in a 0.1 M HClO
4
solution, and calculated
binding energies (circles) of atomic oxygen (BE
O
), as a function of calculated d-band center
(relative to the Fermi level, 1
d
2 1
F
) of the respective surfaces. The data for Pt(111) were
obtained from [Markovic et al., 1999] and are included for comparison. Key: 1, Pt
ML
/
Ru(0001);
2,
Pt
ML
/Ir(111);
3,
Pt
ML
/Rh(111);
4,
Pt
ML
/Au(111);
5,
Pt(111);
6,
Pt
ML
/
Pd(111). (Reproduced with permission from Zhang et al. [2005a].)
unduly hindering either. To identify the fundamental reasons behind the volcano-type
behavior shown in Fig. 9.14, DFT calculations were performed to study the following
elementary steps:
O
2
!
O
þ
O
(9
:
3)
O
þ
H
!
OH
(9
:
4)
These two steps were chosen because they are the most activated versions of O - O
bond scission and O - H bond formation, respectively. There is evidence that the reac-
tivity of other hydrogenated forms of oxygen follows a similar trend to that of atomic
oxygen [Shao et al., 2007a].
The calculated activation energy barrier E
a
for the elementary steps (9.3) and (9.4)
are plotted against O binding energy in Fig. 9.15. The binding energy of O is empha-
sized because atomic oxygen is the common intermediate for (9.3) and (9.4), and inter-
acts more strongly than any other ORR intermediate with the surface, thus making O
adsorption more sensitive to surface properties. Previous studies have shown that sur-
faces that bind an adsorbate strongly tend to enhance the kinetics of bond scission
steps in which the adsorbate is a product. On the other hand, surfaces that bind an

















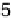




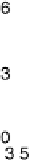


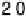


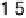































Search WWH ::

Custom Search