Environmental Engineering Reference
In-Depth Information
Figure 9.8 (a) Voltammetry curve of a Au(100) electrode in 0.1 M HClO
4
with 20 mM Br
2
,
at 10 mV/s. (b) X-ray intensity at (1 : 2, 1 : 2, 0.1) position as a function of potential for
Br : Au(100) in the absence (dashed line) and presence (full line) of O
2
,at1mV/s. (c) O
2
reduction on Au(100) (dashed line) and Au /Br (full line), at 20 mV/s. (Reproduced with
permission from Adzic and Wang [2000].)
H
3
O
þ
-SO
22
ion pair, possibly with the three unprotonated sulfate oxygen atoms
interacting with Pt sites. A weak band at 940 cm
21
is assigned to the S - O(H
3
O
þ
)
stretch of the ion pair, and a band at 1040 cm
21
, which disappears with increasingly
more positive sample potentials, is assigned to the symmetric stretching mode for sol-
ution phase bisulfate ions. Chronocoulommetry data also indicate the adsorption of
bisulfates [Savich et al., 1995]. Although there are no structural studies of bisulfate
adsorption during the ORR, the structure of the ordered bisulfate adlayer on submersed
Pt(111) has been found to be (
p
)-R308 [Thomas et al., 1996]. This adlayer can
effectively block the ORR on the Pt(111) surface. Using Monte Carlo methods, Koper
and Lukkien modeled the butterfly peak associated with bisulfate adsorption on
Pt(111) with the latter structure [Koper and Lukkien, 2000].
p

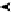
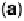
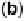


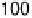


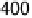





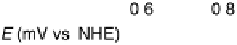
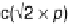
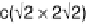




























Search WWH ::

Custom Search