Environmental Engineering Reference
In-Depth Information
1.3 ELECTROCATALYSIS OF OXYGEN REDUCTION IN THE FUEL
CELL CATHODE: NEW INSIGHTS AND NEW QUESTIONS
Significant advances have been made since the year 2000 in the theoretical analysis of
the ORR at Pt metal electrocatalysts. These recent theoretical advances have been
made using DFT calculations considering model systems of good resemblance to
the actual electrochemical interface. Models of the interface used in such recent
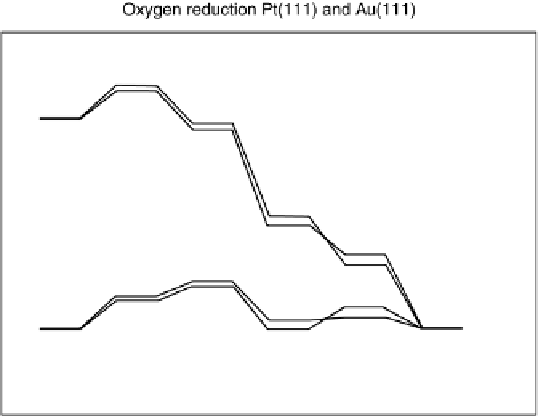
calculations include a significant ensemble of metal atoms (Fig. 1.2) [Nørskov
et al., 2004] (rather than a single metal atom or a pair of metal atoms as was done
in pioneering contributions made somewhat earlier [Anderson et al., 2000]), as
well as the aqueous molecular environment adjacent to the metal catalyst surface.
Last, but not least, the effects of variations in the electrode - electrolyte potential
difference on interfacial thermodynamics and dynamics have also been included in
these models [Nørskov et al., 2004; Panchenko et al., 2004; Desai and Neurock, 2003].
Figure 1.2 Free energy map for the “associative mechanism” of ORR at Pt metal and the steps
considered in this mechanism [Nørskov et al., 2004].






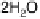

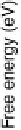








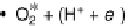



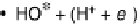

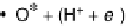
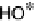

























Search WWH ::

Custom Search