Environmental Engineering Reference
In-Depth Information
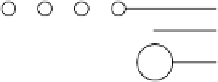
Figure 9.7 Proposed structural models for Ru(0001) oxidation, where the O, S, and Ru atoms
are represented by the open, lightly shaded, and heavily shaded circles, respectively. The inter-
layer distances are in nanometers and coverage in monolayers (ML). (Reproduced with per-
mission from Wang et al. [2001].)
solutions below the onset of bulk oxide formation show that the spacing between the
top two Ru layers is 0.213 nm at 0.1 V, and 0.220 nm at 1.0 V in 1 M H
2
SO
4
solution,
similar to the values in the gas phase for bare Ru and for 1 ML of oxygen on Ru (0.210
and 0.222 nm, respectively) [Wang et al., 2001]. At low potentials, the specular
reflectivity data support a model involving the co-adsorption of bisulfate and hydro-
nium ions on Ru(0001). The coverage of bisulfate is close to
1
3
ML at potentials
below 0.57 V. Figure 9.7 shows the proposed structural models. In contrast to the
behavior of Pt(111) and Au(111) surfaces, no place exchange is involved in
Ru(0001) surface oxidation. The formation of a monolayer of Ru oxide induces partial
desorption of bisulfate, in agreement with the Fourier transform infrared (FTIR) data.
These properties of Ru - OH affect the activity of mixed Pt - M monolayers for the
ORR, as discussed later in this chapter.
9.2.2 Adsorption of Anions
The effects of anions on the kinetics of the ORR are of fundamental interest to
developing an understanding of the relationship between surface structure and
composition and surface activity. Like OH
ads
, strongly (also termed “specifically”)
adsorbed anions can inhibit the ORR and significantly reduce its reaction rate. The
effects are surface structure-dependent, with the inhibition ranging from low to
very high for the same metal. The most pronounced inhibition is caused by halides
(chlorides, bromides, and iodides). Bisulfates/sulfates and phosphates have weaker
adsorption and cause less inhibition; nitrates adsorb more weakly still, while, for all
practical purposes, perchlorates, fluorides, and perfluoroacid anions do not adsorb
[Adzic and Wang, 2000]. Traces of Cl
2
impurities often affect the results with non-
adsorbing anions.
There are several factors through which anions can influence the pathway and O
2
reduction kinetics. The main factors are competition with O
2
for surface sites; changes
in the activity coefficients of the reactants, intermediates, and transition states; and the
acidity and dielectric properties of the electrolyte side of the interface [Adzic, 1998].
For example, perfluoro acids have higher O
2
solubility and lower adsorbability than







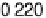
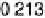
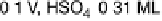
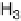

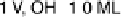


























Search WWH ::

Custom Search