Environmental Engineering Reference
In-Depth Information
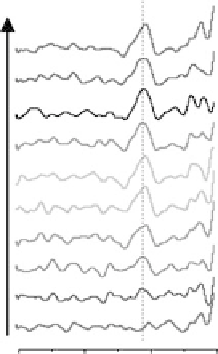
Figure 9.1 High symmetry adsorption sites for O and O
2
on Pt(111). Large gray circles rep-
resent Pt atoms, and small open circles represent O atoms. “t,” “b,” “h,” and “f ” stand for top,
bridge, hcp, and fcc, respectively. The surface unit cell is delineated. (Reproduced with per-
mission from Xu et al. [2004].)
state is ambiguous and is suggested by some authors to be a peroxide state and by
others to be a superoxide state (O
2
2
). The t-b-t state is the most stable, with a binding
energy of 20.6 eV at
4
monolayer (ML).
To gain information on O
2
adsorption on Pt(111) during the course of ORR, Adzic
and Wang used the adsorption of other metal atoms (Ag) as a probe [Adzic and Wang,
1998]. The inhibition of the ORR on Pt(111) by sub- and monolayer coverages of Ag
was studied using electrochemical and in situ surface X-ray scattering techniques. The
analysis of the extent of the inhibition of the ORR as a function of the Ag coverage
showed that the data are best interpreted with O
2
occupying a bridge site. More
recently,
Shao
and
co-workers
used
surface-enhanced
infrared
reflection
and
Figure 9.2 SEIRAS spectra for a Pt thin film electrode in O
2
-saturated 0.1 M NaClO
4
þ
NaOH ( pH 11). The reference spectrum at 0.4 V was taken before the potential sweep started.
The scan rate was 10 mV/s. (Reproduced with permission from Shao et al. [2006a].)










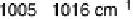




























Search WWH ::

Custom Search