Environmental Engineering Reference
In-Depth Information
Pt, the second atomic layer is found to be depleted in Pt (48 at% of Pt), the third layer is
again enriched in Pt (87 at%), and, beyond that, the bulk value of 75 at%. It is clear
from these results that the Pt-skin(111) surface is stable in UHV, during transfer
and in the electrochemical environment. It is also apparent from X-ray voltammetry
(XRV) (Fig. 8.13a) that the relaxation of both surfaces is induced by OH
ad
and
hence the potential shift is consistent with the cyclic voltammogram. The Pt-skin sur-
face is stable over a wider range than the Pt(111) surface, perhaps owing to the
increased contraction and the lower coverage by OH
ad
, which is the precursor to irre-
versible oxide formation as well as dissolution. On this basis, it was possible to draw
the conclusion that the Pt-skin surface is more stable owing to a less pronounced inter-
action with surface oxides. The stability of such surfaces in an electrochemical
environment was relatively unknown, and so these findings were crucially important
for potential applications of these surfaces as electrocatalysts.
The observed oscillatory concentration profile is the first instance of segregation
profiles being monitored at different potentials in electrochemical environment and
it was in excellent agreement with the results previously obtained on similar single-
crystalline systems in UHV by Gautier and co-workers [Gauthier, ]. Since this type
of segregation is characteristic of the first four atomic layers, we term these systems
surfaces with a nanosegregated profile. Further electrochemical characterization
revealed the unique behavior of these surfaces. In Fig. 8.14, a representative cyclic vol-
tammogram of Pt
3
Ni(111)-skin is compared with a Pt(111) surface. The two electro-
des have the same surface composition and structure of the topmost layer, but different
compositions of the subsurface layer and hence different electronic structures. This
leads to dramatic differences in their adsorption properties, which are nicely visible
Figure 8.14 Cyclic voltammetry of Pt
3
Ni(111) and Pt(111) surfaces in 0.1 M HClO
4
,at30mV/s.
(Reprinted with permission from Stamenkovic et al. [2007a]. Copyright 2007. American
Association for the Advancement in Science.)



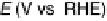
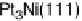













































































































































































































































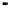



































































































































































































































































































































































































































































































































































































































































































































































Search WWH ::

Custom Search