Environmental Engineering Reference
In-Depth Information
Figure 8.7 (a) Cyclic voltammograms of Pt(111) (dashed gray line) and Pt
3
Sn(111) (solid black
line) in 0.5 M H
2
SO
4
;scanrate50mV/s. Potential-dependent integrated charges for the adsorption
of (bi)sulfate anions on the Pt
3
Sn(111) surface are represented by circles. (b) Measured X-ray inten-
sities at (1, 0, 3.7) and (1, 0, 4.3) as functions of electrode potential. The ball models show top and
side views of the p(2
2) structure: gray circles, Pt atoms; black circles, Sn atoms; triangles,
(bi)sulfate anions adsorbed on Pt sites. The side view indicates the surface normal spacing that
is derived from the crystal truncation rod (CTR) measurements. (Reprinted with permission
from Stamenkovic et al. [2003]. Copyright 1999. The American Chemical Society.)
The results indicate two important characteristics of the surface structure at 0.05
and 0.55 V: (i) at 0.05 V, expansion of the Pt surface atoms induced by the adsorption
of hydrogen is very similar to that observed on Pt(111) [Janssen et al., 2004; Iwasita
and Nart, 1997] (Dd
Pt
¼þ
2%); (ii) at 0.55 V, the p(2
2) structure remains stable
but, while the Pt surface atoms are unrelaxed, the Sn atoms in the topmost layer
expand up to (Dd
Sn
¼
8
:
5%) of the lattice spacing. At potentials higher than 0.55 V,
this expansion is even more pronounced, indicating that before Sn dissolution, the
Sn surface atoms are expanded by approximately 12% of the bulk lattice spacing.
Cyclic voltammetry confirms the presence of non-Pt atoms on the surface by a signifi-
cantly attenuated hydrogen adsorption/desorption region, as well as characteristic
adsorption of anion (sulfate) on Pt sites, which is represented by a reversible voltam-
metric feature at about 0.35 V (Fig. 8.7a). These in situ results confirmed that the




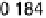

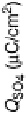

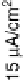



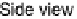
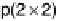

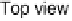


















































Search WWH ::

Custom Search