Environmental Engineering Reference
In-Depth Information
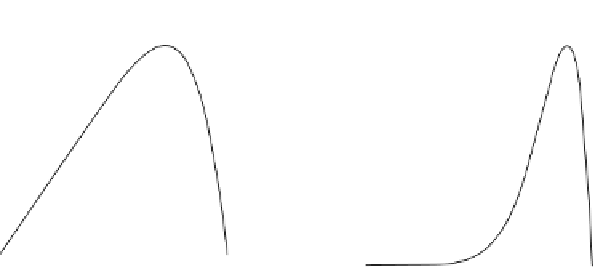
Figure 7.14 Current density for formic acid oxidation as a function of the fraction of Pt sur-
face atoms blocked by adatoms on two different electrodes: (a) Bi/Pt(111); (b) Sb/Pt(100).
(Reprinted with permission from Leiva et al. [1997].)
very steep decrease at coverages near saturation, with a maximum current density for a
blockage of around 70%. This shape has been explained with a very simple model
[Leiva et al., 1997] that considers that the adatoms play an electronic effect in such
a way that the current density for a given coverage is proportional to the number of
Bi - Pt pairs (i.e., the number of Bi atoms that have at least one adjacent unblocked
Pt atom). The initial linear increase supports this model, since, for low adatom cover-
age, each deposited adatom contains at least one neighboring free Pt atom. In this
model, the activity of each adatom is the same, regardless of the number of unoccupied
sites next to it. For high adatom coverages, the availability of free sites starts to
decrease and, with it, the activity towards the direct oxidation reaction. The position
of maximum activity can be calculated from probabilistic considerations, being
located at 72% occupied Pt sites. The agreement between the model and the exper-
imental results is excellent.
The situation is more complex when the poisoning reaction takes place at the same
time as the direct oxidation and the poison acts as a blocking agent for the direct path-
way. In this case, the result depends on the degree of poison formation. One extreme
case is when all available sites are blocked by the poison. This is the case with
Sb-Pt(100), depicted in Fig. 7.14b. The shape of the curve in this figure has been
explained by considering that adatoms exert an ensemble effect and that poison for-
mation can only take place if at least two adjacent Pt atoms are available [Leiva
et al., 1997]. Then, free Pt sites completely surrounded by adatoms will not be
active for the poison formation but will be active for the direct oxidation. For low cov-
erages, the activity remains very low, since the probability of having a Pt site comple-
tely surrounded by adatoms will be very low. The current only starts to increase for
blockage degrees higher than 0.5, featuring a maximum at about 0.9, in excellent
agreement with the theoretical model.


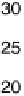


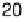




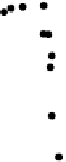

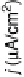
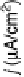





















Search WWH ::

Custom Search