Environmental Engineering Reference
In-Depth Information
in [Blais et al., 2002] was to correct the value of Bi(OH)
3
with a factor of 2/3. In this
way, reasonable coincidence is observed between the enthalpy of formation of the sur-
face hydroxide in the electrochemical environment (DH
f
¼
440 kJ
=
mol) and the
formation of the bulk hydroxide (DH
f
¼
474 kJ
=
mol), supporting the interpretation
of the voltammetric data.
Although, for other adatoms, the study of the temperature dependence of the peak
potential has not yet been performed, it is still worth analyzing the relationship
between the peak potential of the adatom redox process and the energy of the bond
between the element and the oxygen or hydroxyl species. Figure 7.4 plots peak poten-
tial values of adatom redox processes on Pt(111) and Pt(100) electrodes against the
enthalpy of formation of the corresponding bulk oxide [Dean, 1999]. In this graph,
the distinction between the formations of oxide or hydroxide species has not been con-
sidered, in order to compare results for different adatoms. Following the procedure in
[Blais et al., 2002], when the oxidation state of the adatom on the Pt surface is not
stable in the bulk solid state, the enthalpy values have been corrected by the ratio
between the oxidation state in the electrochemical environment and in the bulk
oxide. This is the case for Bi
2
O
3
,Sb
2
O
3
,Pb
3
O
4
, and GeO
2
.
It is observed that higher potential values for the adatom redox process are
correlated with a lower energy of the M22O bond, i.e., lower (less negative) enthalpy
of formation of the adatom oxygenated species. In this regard, the discrepant behavior
of Ge-Pt(100) may be related to the dilute nature of this adlayer, with a maximum
coverage of only 0.25.
Figure 7.4 Peak potential values of adatom redox processes on Pt(111) and Pt(100) electro-
des in 0.5 M H
2
SO
4
solution, as labeled, plotted against the enthalpy of formation of the corre-
sponding bulk oxide. Lines are included to indicate the tendency (the full line corresponds to the
filled squares, and the dashed line to the open circles).
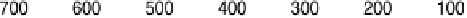














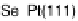

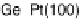

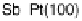
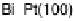

































Search WWH ::

Custom Search