Environmental Engineering Reference
In-Depth Information
both processes). Although, a priori, this assumption seems arbitrary, it has been proven
correct in many situations. Besides, in the particular case of Pt(111) in H
2
SO
4
sol-
utions, it has been shown that q
u
¼
0
Pt
¼
(q
u
¼
0
H
þ
q
u
¼
0
An
)
¼
q
Pt(hkl)
[Feliu et al., 1994],
and hence (7.11) simplifies to
n
q
Ad
)
q
u
¼
0
m
¼
q
Pt
þ
m
q
Pt
¼
q
u
¼
0
n
q
Ad
(7
:
12)
Pt
Pt
On the other hand, in certain cases (e.g., Ad/Pt(111) in HClO
4
solutions), the adatom
oxidation and anion adsorption on the free sites (OH adsorption in HClO
4
) overlap.
Then, another refinement to this analysis would be to use (7.7) and (7.10) to calculate
q
An
according to
q
H
q
u
¼
0
H
q
An
¼
q
u
¼
0
(7
:
13)
An
and then subtract this charge density from the total charge density measured under the
adatom peak [Climent et al., 2006].
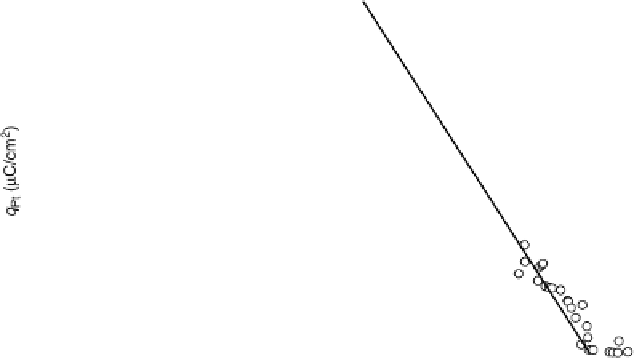
According to (7.8) and (7.12), the stoichiometry (m/n) can be extracted from the
slope of the plots of adatom charge density versus hydrogen (or hydrogen plus
anion) charge density. Some representative plots are shown in Fig. 7.3. The con-
clusions extracted from this kind of analysis are summarized in Tables 7.1 and 7.2
for Pt(111) and Pt(100) modified surfaces, respectively. The extension of this analysis
Figure 7.3 Plot of the platinum (hydrogen plus anion) charge density versus the charge den-
sity associated with the adatom redox process (Bi or Te, as indicated) on a Pt(111) electrode in
0.5 M H
2
SO
4
solution. Straight lines represent the expected behavior for the stoichiometry
indicated in the figure.


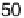
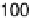

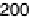
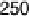


















Search WWH ::

Custom Search