Environmental Engineering Reference
In-Depth Information
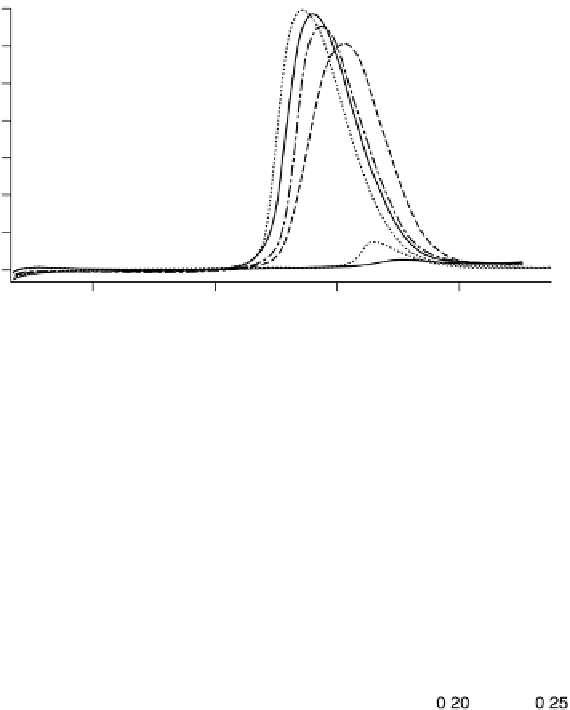
Figure 6.22 (a) Cyclic voltammograms of the oxidation of 0.1 M acetaldehyde on Pt single-
crystal electrodes in 0.5 M H
2
SO
4
at a scan rate of 10 mV/s. (b) Dependence of the peak poten-
tial (upper) and maximum current density (lower) of acetaldehyde oxidation on the step density.
The solid lines are the least squares fit of the data.
6.6 CONCLUSIONS
In this chapter, we have summarized (recent) progress in the mechanistic understand-
ing of the oxidation of carbon monoxide, formic acid, methanol, and ethanol on
transition metal ( primarily Pt) electrodes. We have emphasized the “surface science
approach” employing well-defined electrode surfaces, i.e., single crystals, in combi-
nation with surface-sensitive techniques (FTIR and online DEMS), kinetic modeling
and first-principles DFT calculations.
CO oxidation is a highly structure-sensitive reaction that needs steps and defect
sites. Mobility of CO on the electrode surface does not seem to play a role on







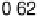
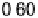
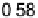



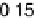



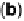


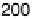
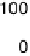
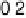
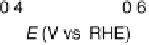
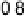
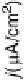

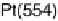


























Search WWH ::

Custom Search