Environmental Engineering Reference
In-Depth Information
of poisoning increases with increasing step density. Fitting of transients with (6.18)
indeed suggested that both k
dec
and k
ox
depend on step density, with Pt(111) having
a much lower decomposition rate constant than Pt(554) and Pt(553), although the
model is probably not accurate enough to establish a clear linear relationship between
rate constant and step density. It was also found that decomposition is typically
faster at low potentials (,0.6 V vs. RHE) and oxidation is faster at high potentials
(.0.6 V vs. RHE).
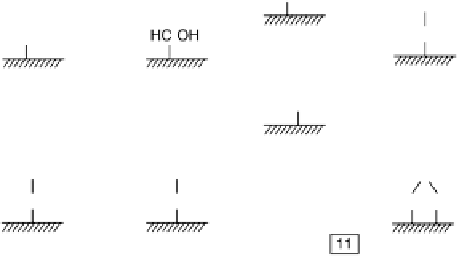
From the results described above, a more detailed reaction scheme has been
suggested, which is summarized in Fig. 6.21. As in the mechanism of Cao and co-
workers [Cao et al., 2005], the decision between the direct and indirect pathway is
made at the initial dehydrogenation step in the approach of the methanol to the surface.
The indirect path (Reactions 1 - 7 in Fig. 6.21) is initiated by the dehydrogenation of
methanol to hydroxymethyl, which is further dehydrogenated to a C/O/H species, the
nature of which is still unclear, and eventually to CO
ads
, which acts as a surface poison
at low potentials. Online electrochemical mass spectrometry (OLEMS) results
[Housmans et al., 2006] indicate that the indirect pathway is favored on (111) terraces
in the absence of a strongly adsorbing anions and on the Pt(100) plane. The direct
pathway, reactions 8 - 15, is initiated by O22H scission to form methoxy, a reaction
known to occur readily under UHV conditions [Kizhakevariam and Stuve, 1993].
Under electrochemical conditions, the OLEMS data suggest that methoxy formation
is preferred on Pt(111) in H
2
SO
4
media, on Pt surfaces with (110) steps in the absence
of (bi)sulfate and on Pt(110) regardless of the electrolyte. The DFT calculations by
Cao and co-workers indicate that dehydrogenation to methoxy is also preferred on
(100) steps [Cao et al., 2005]. The formed methoxy is further dehydrogenated to
Figure 6.21 Suggested reaction scheme for the electrochemical oxidation of methanol on
metal electrodes. (After Housmans et al. [2006].)


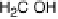
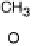















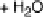


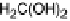
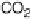
































Search WWH ::

Custom Search