Environmental Engineering Reference
In-Depth Information
whereas formic acid and formaldehyde yields are not affected [Batista et al., 2003,
2004]. A detailed study of the product distribution on a series of stepped single-crystal
Pt electrodes using online electrochemical mass spectrometry revealed that steps
influence not only the activity of methanol oxidation, but also the reaction paths
chosen [Housmans et al., 2006] (Fig. 6.19). In HClO
4
(i.e., in the absence of strongly
adsorbing anions), steps promote the formation of methyl formate, which is produced
by the reaction of methanol with the oxidation intermediate formic acid. Pt(110) pro-
duces about 4 - 5 times more methyl formate than Pt(111). This suggests that the direct
path via formic acid is catalyzed by steps and defects. In H
2
SO
4
, the same trend
with step density is observed, but Pt(111) is nevertheless the most active surface in
producing methyl formate. Pt(111) is also the only surface that produces more
methyl formate in H
2
SO
4
than in HClO
4
. This suggests that anion adsorption on the
(111) terrace also favors the direct pathway.
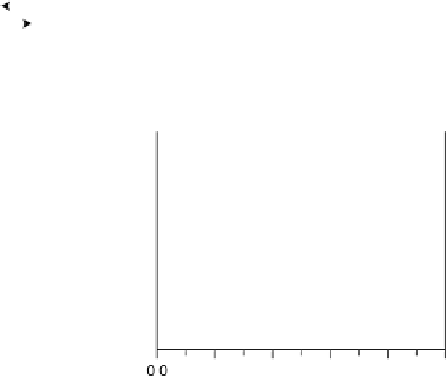
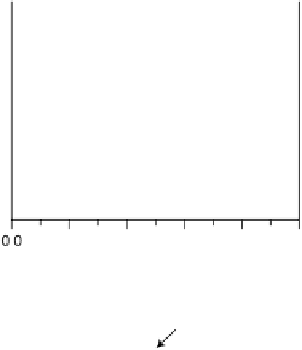
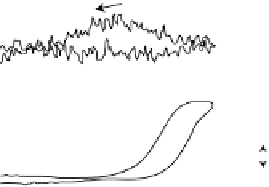
Figure 6.19 (a) Cyclic voltammograms of Pt(111) (solid line), Pt(110) (dashed line), and
Pt(100) (dotted line) in 0.5 M CH
3
OH and 0.5 M H
2
SO
4
at a scan rate of 2 mV/s. The inset
shows a zoom of the Pt(111) cyclic voltammogram. The top mass shown in the associated
mass spectroscopic cyclic voltammograms for (b) Pt(111), (c) Pt(110), and (d) Pt(100) displays
the methyl formate signal, which is associated with the formic acid produced in the reaction
(m/z ¼ 60), and the bottom one displays the CO
2
signal (m/z ¼ 44). (Reproduced from
Housmans et al. [2006].)
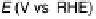

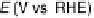
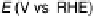





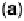
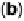




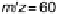











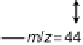





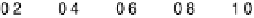


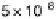


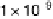
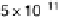

























































Search WWH ::

Custom Search