Environmental Engineering Reference
In-Depth Information
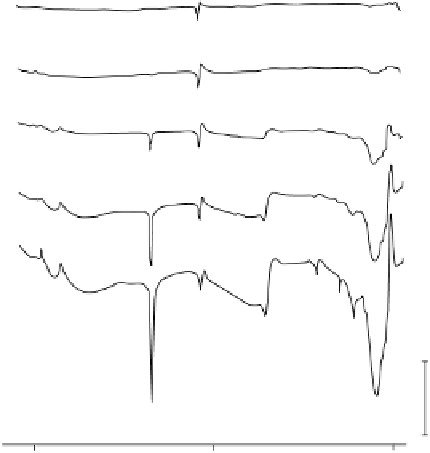
Figure 6.18 Subtractively normalized interfacial Fourier transform infrared spectroscopy
(SNIFTIRS) spectra of a polished polycrystalline Pt electrode, immersed in 0.1 M HClO
4
,
þ
5MCH
3
OH electrolyte. All spectra were normalized to the base spectrum collected at 0 mV
vs. RHE. (Reproduced from Iwasita and Vielstich [1988].)
for Pt(111) electrodes is this contribution through the non-CO path important [Herrero
et al., 1995]. The formation of formic acid on a polycrystalline Pt electrode has also
been detected indirectly by attenuated total reflection (ATR) spectroscopy, since its
adsorption product, formate, has been detected during the oxidation of methanol,
which supports the dual pathway mechanism for any Pt electrode [Chen et al.,
2003]. The formation of intermediate oxidation products (formaldehyde and formic
acid) reveals that the oxidation of methanol is not complete, and this is one of the
reasons for the low currents detected, since the average number of electrons exchanged
per methanol molecule is always smaller than 6.
The amounts of formaldehyde and formic acid have been measured for several Pt
single crystals [Batista et al., 2003, 2004; Housmans et al., 2006; Wang and
Baltruschat, 2007]. The fraction of methanol molecules oxidized to CO
2
is about
20% at 0.60 V and 32% at 0.80 V, with formic acid and formaldehyde being the
remaining oxidation products [Wang and Baltruschat, 2007].
Increasing the temperature seems to facilitate the oxidation of CO, since lower
amounts are accumulated on the surface [Kardash and Korzeniewski, 2000]. The pre-
sence of strongly adsorbed anions in the electrolytic solution affects the distribution of
products. It has been shown that adsorbed (bi)sulfate diminishes the production of CO,
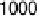
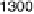
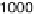

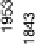
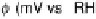
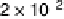
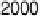





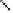











Search WWH ::

Custom Search