Environmental Engineering Reference
In-Depth Information
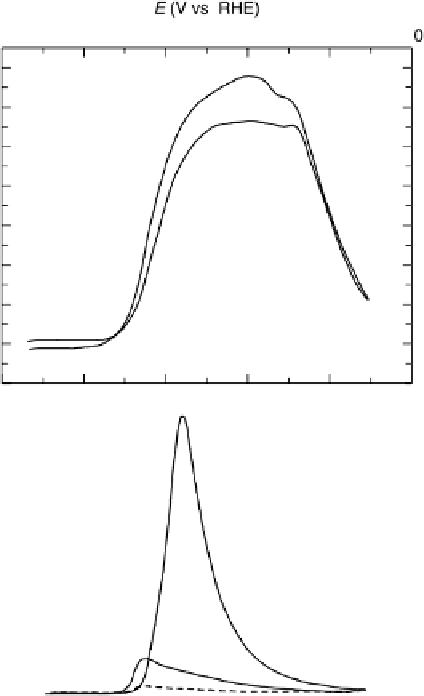
Figure 6.13 Voltammetric profile for (a) Pt(111) and (b) Pt(100) electrodes in 0.1 M
HCOOH
þ
0.5 M H
2
SO
4
on electrode. The full lines show the first cycle and the dashed line
the second cycle. The scan rate was 50 mV/s.
revealed by the blocking of the hydrogen adsorption states, and its oxidation at high
potentials. Thus, in the return scan, the platinum surface is completely clean, and
therefore the catalytic activity of the surface is much higher than that recorded for
the positive scan.
This general scheme for the oxidation reaction is very sensitive to the surface
structure. The first studies with single-crystal electrodes revealed that the voltammetric
profiles for the three basal planes, Pt(111), Pt(100), and Pt(110), were completely
different (Fig. 6.13) [Clavilier et al., 1981; Lamy et al., 1983; Adzic et al., 1982].
The lowest currents are obtained for the Pt(111) electrode, which in turn has a very
low poisoning rate, as suggested by the small hysteresis. In fact, the reaction on this











































Search WWH ::

Custom Search