Environmental Engineering Reference
In-Depth Information
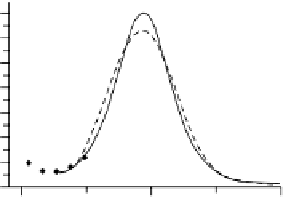
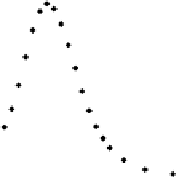
Figure 6.2 (a) Stripping chronoamperometry current transients for the oxidation of a saturated
CO monolayer on Pt(553), Pt(554), and Pt(15, 15, 14) in 0.5 M H
2
SO
4
. The potential is 0.855 V
with respect to RHE. (b) Fit of a current transient obtained at Pt(15, 15, 14) for E ¼ 0.88 V. The
diamonds are experimental data points, the solid line is the best fit by the mean field model, and
the dashed line is the best fit by the N&G model.
and no tailing in the transient is observed on the electrodes with wide terraces, such as
Pt(15, 15, 14) or Pt(111). Both observations strongly suggest that the mobility of CO
on the (111) terraces must be considered high.
From the fit of the experimental transient, the rate constant k
hkl
(E) can be obtained
for the various electrode surfaces at a series of electrode potentials. Figures 6.3 and 6.4
plot the rate constant as a function of the step density at various potentials, and the log-
arithm of the rate constant as a function of potential for the different surfaces (Tafel
plot). The inset in Fig. 6.3 plots the “intrinsic” rate constant, i.e., the measured rate
constant divided by the step density, and, together with the main plot in Fig. 6.3,
these data demonstrate that the measured rate is proportional to the step density.
Therefore, the reaction takes place exclusively at the steps, and, in the potential
range studied, the Pt(111) terraces must be considered inactive for CO oxidation.
The Tafel slopes obtained from Fig. 6.4 are all very close to 70 - 80 mV/dec, in
good agreement with earlier measurements of the Tafel slope for CO oxidation
[Love and Lipkowski, 1988; Palaikis et al., 1988; Bergelin et al., 1999]. We interpret
this Tafel slope as an “EC” mechanism, in which an electron transfer reaction is in
equilibrium, followed by an essentially potential-independent chemical step:
H
2
O
þ
!
OH
ads
þ
H
þ
þ
e
equilibrium
(6
:
8)
CO
ads
þ
OH
ads
!
COOH
ads
rate-determining step
(6
:
9)
COOH
ads
!
CO
2
þ
H
þ
þ
e
þ
2
(6
:
10)
Shubina and co-workers calculated the activation energy for the reaction between
CO and OH on a Pt(111) surface in the absence of water, and obtained a value of about
0.6 eV [Shubina et al., 2004]. Janik and Neurock [2007] calculated the barrier for this
reaction on Pt(111) in the presence of water and as a function of the surface charge
of the Pt(111) electrode. They found a value of 0.50 eV in the absence of a surface
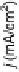






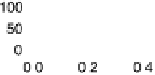
























Search WWH ::

Custom Search