Environmental Engineering Reference
In-Depth Information
and relevance of these surface science studies employing single crystals with respect
to the same reactions studied on more realistic catalytic surfaces consisting of small
nanometer-sized particles on a carbon support. This review will not be exhaustive,
and will mainly focus on results obtained in our own research groups. Nevertheless,
we will point out the relation to similar work done in other groups as much as possible.
The interested reader is also advised to consult literature reviews from other groups
dealing with similar topics [Parsons and VanderNoot, 1988; Beden et al., 1992;
Jarvi and Stuve, 1998; Sun, 1998; Markovic and Ross, 2002; Lai et al., 2008].
The electrode kinetics of the reactions considered in this chapter on bimetallic and
nanoparticulate surfaces is discussed in detail in other chapters in this volume (e.g.,
Chapters 8, 10, and 15).
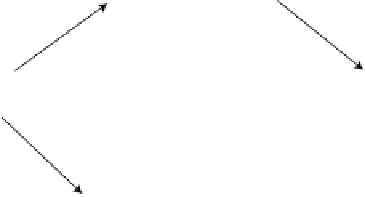
Perhaps the most important paradigm in research on the mechanism of the
electrocatalytic oxidation of small organic molecules is the dual pathway mechanism
introduced in Capon and Parsons [1973a, b], and reviewed in Parsons and VanderNoot
[1988]. In terms of methanol oxidation, the dual pathway may be summarized in a
simplified way by Fig. 6.1. The idea is that the complete oxidation of methanol to
carbon dioxide may follow two different pathways:
†
A so-called indirect pathway involving a strongly adsorbed intermediate. This
intermediate is now commonly accepted to be CO. The sluggishness of this path-
way has much to do with difficulty of oxidizing of this strongly adsorbed CO,
explaining the importance of understanding the catalysis of CO oxidation. The
oxidation of CO is the topic of Section 6.2.
†
A so-called direct pathway involving a more weakly adsorbed perhaps even
partially dissolved intermediate. Likely candidates for such intermediates are for-
maldehyde and formic acid. The oxidation mechanism of formic acid is discussed
in Section 6.3. The idea is that the formation of a strongly adsorbed intermediate
is circumvented in the direct pathway, though in practice this has appeared difficult
to achieve (the dashed line in Fig. 6.1). Section 6.4 will discuss this in more detail in
relation to the overall reaction mechanism for methanol oxidation.
Figure 6.1 Schematic representation of the “dual pathway” mechanism for the electrocatalytic
oxidation of methanol to carbon dioxide.


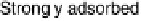
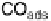
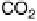

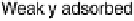











Search WWH ::

Custom Search