Environmental Engineering Reference
In-Depth Information
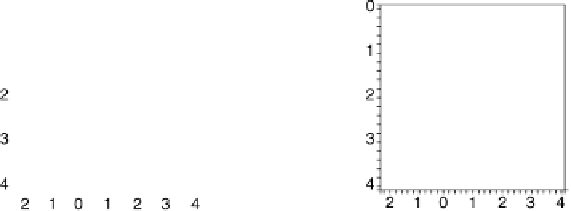
Figure 5.11 (T, a, Df) phase diagrams for all low index surfaces of a-PtO
2
(a) and b-PtO
2
(b). Each plot shows the thermodynamically most stable surface structures as functions of
Dm
H
2
O
and Df. In addition, the temperature scale corresponding to a
H
2
O
¼
1(p ¼ 1 atm) is
shown on the right side of each phase diagram. The surface models are named with their orien-
tations and surface terminations.
potentials are rather different. At room temperature and an electrode potential around
1.2 V, which corresponds exactly to the transition of the phase-separated system and
the bulk oxide, the (110) surface with bulk composition is again most stable, showing
only minor deviations from the corresponding bulk-truncated structure. A slight
increase in the electrode potential stabilizes the same surface orientation, but with
an additional oxygen atom per unit cell, until approximately 1.4 V. Induced by
these excess atoms, which are not compensated by corresponding platinum atoms,
structural changes on the surface can be observed such that adjacent oxygen atoms
start forming bonds. The resulting O22O bond length is comparable to the distance
in the OOH radical and H
2
O
2
molecule [Jacob, 2006a, b], indicating a single covalent
bond. Above 1.4 V, a second excess oxygen per surface unit cell is stabilized. As a
result, two of now three excess oxygens form an O55O double bond.
This example describes how the extended ab initio atomistic thermodynamics
approach can reveal further insights into the growth of the oxide. It has been shown
that at electrode potentials that are not too high, oxide growth proceeds by first forming
a thin a-PtO
2
(001) surface oxide with a PtO composition. Further growth then results
in an a-PtO
2
(001) or b-PtO
2
(110) bulk oxide. This finding agrees with experimental
observations along the oxidation mode [Gilroy and Conway, 1968; Allen et al., 1974;
Tremiliosi-Filho et al., 1992], but in addition specifies the corresponding surface
structures. Along the reduction mode, Tremiliosi-Filho et al. [1992] found the oppo-
site growth mechanism. Although this seems to be in contrast with our results, one has
to consider that the surface structures discussed here correspond to thermodynamically
stable phases. Thus, any energetic barriers that the system has to overcome in order to













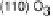


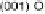














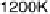
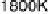

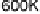
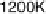
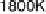
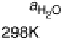



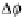

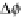

























Search WWH ::

Custom Search