Environmental Engineering Reference
In-Depth Information
here and to better understand the role of the electrode potential, in the following we
will first consider a system in which the electrolyte is absent, and afterwards address
the electrochemical system.
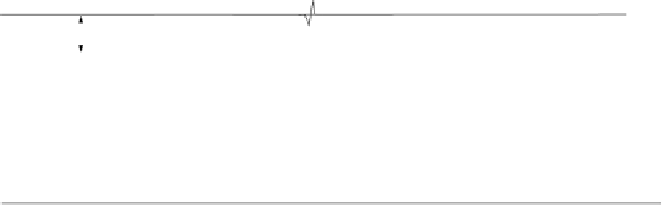
Figure 5.3 shows a system of two electrodes of the same material, which is com-
parable to a capacitor in classical electrostatics, and the shape of the electrostatic
potential before and after applying an external voltage bias, i.e., a charged capacitor.
In the absence of an external voltage, the capacitor is uncharged. The electrostatic
potential fwithin each metallic electrode is determined by the charge of the nuclei,
which gives rise to singularities at the positions of the atoms, and by the electrons.
At the surface, the behavior is additionally influenced by the broken periodicity, lead-
ing to a so-called surface dipole [Horn and Scheffler, 2000]. Moving from the metal
into the vacuum, the electrostatic potential finally approaches the vacuum level f
vac
(which is the same for each electrode of an uncharged capacitor). In the following,
we define this value as reference potential f
ref
, which, without loss of generality,
we have already chosen as the energy zero for the electrostatic potential.
The energy required to move an electron from deep inside the electrode into the
vacuum is called the work function F. It allows us to define the (electro)chemical
potential of the electrons, which is the energy change when removing one electron
Figure 5.3 Electrostatic potential f in a capacitor without (a) and with (b) an externally
applied potential difference. Both electrodes are assumed to be of the same material.


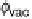
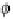
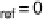

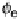































Search WWH ::

Custom Search